Introduction
Medicines optimisation is a person-centred approach to safe and effective medicines use, to ensure people obtain the best possible outcomes from their medicines (NICE 2015). By focusing on patients and their experiences, medicines optimisation aims to help patients to: improve their outcomes, take their medicines correctly, avoid taking unnecessary medicines, reduce wastage of medicines and improve medicines safety (RPS 2013). Psychotropic medicines, namely antidepressants, antipsychotics and mood stabilisers, are the main pharmacological treatments for severe mental illnesses (SMIs) such as schizophrenia, psychosis, schizoaffective, bipolar and major depressive disorders (NHS 2018; Maidment et al. Reference Maidment, Sud and Chew-Graham2022). However, such medicines have potentially significant side-effects resulting in poor adherence to treatment and physical morbidity, and such a population has a significantly increased risk of premature mortality (up to 30 years earlier than the general population) (DE Hert et al. Reference DE Hert M, Correll, Bobes, Cetkovich-Bakmas, Cohen, Asai, Detraux, Gautam, Möller, Ndetei, Newcomer, Uwakwe and Leucht2011). Thus, to ensure adequate control of symptoms of mental illness, while avoiding serious complications and negative physical health outcomes, psychotropic medicines must be optimised (Carolan et al. Reference Carolan, Keating, Strawbridge and Ryan2019).
The specialist mental health pharmacist (SMHP) working in the inpatient mental health setting can provide leadership and support for medicines optimisation. People with SMI are less likely to have regular contact with a general medical provider and receive routine physical health screening, monitoring or interventions (Carolan et al. Reference Carolan, Keating, McWilliams, Hynes, O’Neill, Boland, Holland, Strawbridge and Ryan2022). As a result, their time as an inpatient in a mental health setting is an ideal opportunity to optimise both physical and mental health medicines use. The SMHP can provide integral contributions to screening, initiating and maintaining treatments, improving medicines adherence, providing medicines information as well as supporting self-advocacy (El-Den et al. Reference El-Den, McMillan, Wheeler, Ng, Roennfeldt and O’Reilly2020).
There is presently limited understanding of what a baseline clinical pharmacy service in a mental health setting looks like. Here we describe a medicines optimisation service provided by a SMHP to three inpatient mental health teams from a community mental health service in Ireland with a catchment area population of over 175,000 with approximately 230 hospital admissions annually. This service is carried out weekly following SMHP review of medication charts, electronic patient records, progress notes and laboratory results and interventions are relayed to the treating team via a structured internal email. By describing this medicines optimisation service and the evidence behind it, we aim to provide a framework for pharmacist-led medicines optimisation (SJOG Medicines Optimisation Checklist© available in supplementary material) that is scalable and adaptable to other settings where those with SMI may be treated.
Patient-facing interventions
Medicines reconciliation on admission to hospital
The medicines optimisation process begins at the very start of the hospital journey with the medicines reconciliation (med-rec). Med-rec is the process of creating the most accurate list possible of all medicines a patient is taking and comparing it to the clinician’s admission/transfer/discharge order, with the goal of providing correct medicines to the patient (Midelfort 2011). Those with SMI are often prescribed multiple medicines for both mental and physical health problems, and complex regimes including restricted access/higher risk medicines (e.g. clozapine, lithium, Monoamine oxidase inhibitors (MAOI), long-acting antipsychotic injections). As a result, they are particularly at risk of medicines discrepancies on admission to hospital (Wheeler et al. Reference Wheeler, Scahill, Hopcroft and Stapleton2018). Psychiatrists are more likely to be involved in overseeing psychotropic medicines, while GPs are expected to manage physical health medicines (Wheeler et al. Reference Wheeler, Scahill, Hopcroft and Stapleton2018). This disconnect is compounded by poor adherence to medicines meaning an accurate reflection of what the patient was actually taking prior to admission is difficult to achieve (Lacro et al. Reference Lacro, Dunn, Dolder, Leckband and Jeste2002; Lambert et al. Reference Lambert, Conus, Eide, Mass, Karow, Moritz, Golks and Naber2004; Bauer et al. Reference Bauer, Glenn, Alda, Bauer, Grof, Marsh, Monteith, Munoz, Rasgon, Sagduyu and Whybrow2019; Dell’Osso et al. Reference Dell’Osso, Albert, Carrà, Pompili, Nanni, Pasquini, Poloni, Raballo, Sambataro, Serafini, Viganò, Demyttenaere, McIntyre and Fiorillo2020).
As med-rec reduces medication errors and improves patient outcomes, it is an important first step in the medicines optimisation process (Wheeler et al. Reference Wheeler, Scahill, Hopcroft and Stapleton2018). Given that the patient is the one ‘constant’ during a transition of care, a patient-centred approach to medicines reconciliation is essential (Wheeler et al. Reference Wheeler, Scahill, Hopcroft and Stapleton2018). The SMHP plays a leading role in the med-rec process by linking directly with the patient and/or their community pharmacy. This facilitates corroboration of admission medicines against those dispensed to/taken by the patient prior to admission, referral/transfer information, admission notes and outpatient prescriptions. Omissions or discrepancies are communicated to the clinician and followed up by the SMHP. A med-rec is completed for all new admissions within three working days of coming to hospital. And so the pharmacist-led medicines optimisation journey begins.
Medicines information: patient information leaflets and medicines lists
The provision of information on the indications and possible side-effects of psychotropic medicine is an important aspect of medicines optimisation as it is linked to improved adherence to treatment and supports shared decision making (Daltroy et al., Reference Daltroy, Katz, Morlino and Liang1991; Mitchel and Selmes Reference Mitchell and Selmes2007). It is also a regulatory requirement in Ireland and is in line with international guidelines; however, it remains suboptimal (Barnes et al. Reference Barnes2011; NICE 2014; CQC 2022; MHC 2023). The SMHP can provide independent education and advice about medicines, including information on the likely benefits and possible side-effects, to those in an inpatient mental health setting (NAPICU 2014). Patient information leaflets (PILs) contain all of the relevant information on psychotropic medicine and are provided by the SMHP directly or via nursing staff with pharmacist details included to all new admissions and to those newly commenced on a psychotropic medicine.
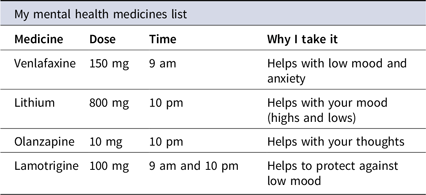
While PILs are suitable for those who are aware and familiar with their diagnosis, the indication described may not be acceptable for some. Those without a formal diagnosis, prescribed psychotropic medicine outside the licensed indication, who lack insight into their condition or who feel stigmatised/marginalised by a mental health diagnosis may also benefit from information on the symptoms the medicine is helping with (Timimi Reference Timimi2014). The ‘Medicines List’ (Table 1) details the medicine’s name, dose, time(s) of administration and targeted symptoms and is provided to all inpatients approximately 2 weeks into their hospital stay. As patients who understand the purpose of a medicine are twice as likely to take it, than those who do not, providing information on targeted symptoms in conjunction with the PIL is an additional step in optimising medicines use (Daltroy et al. Reference Daltroy, Katz, Morlino and Liang1991).
Table 1. My medicines list
Assessing antipsychotic side-effects
Antipsychotic medicines are frequently prescribed to those with SMI and are associated with a varying range of subjectively unpleasant side-effects including sedation, weight gain, constipation, urinary incontinence, breast milk production, erectile and sexual dysfunction. As a result, many do not take their medicines effectively and the prevalence of non-adherence is high – up to 80% of those prescribed antipsychotic medicine discontinue prematurely (Lacro et al. Reference Lacro, Dunn, Dolder, Leckband and Jeste2002, Lambert et al. Reference Lambert, Conus, Eide, Mass, Karow, Moritz, Golks and Naber2004). The impact of non-adherence on patient outcomes is marked and it is one of the most powerful predictors of relapse resulting in rehospitalisation and longer hospital admission (Velligan et al. Reference Velligan, Weiden, Sajatovic, Scott, Carpenter, Ross and Docherty2009, Marder Reference Marder2013). In effect, there is a five-fold increase in relapse rates amongst those with schizophrenia and a five-fold increase in suicide rates amongst those with bipolar disorder that do not adhere to medicines (Gonzalez-Pinto et al. Reference Gonzalez-Pinto, Gonzalez, Enjuto, Fernandez de Corres, Lopez, Palomo, Gutierrez, Mosquera and Perez de Heredia2004; Velligan et al. Reference Velligan, Weiden, Sajatovic, Scott, Carpenter, Ross and Docherty2009). Non-adherence is mainly determined, among other factors, by negative attitudes towards antipsychotic medicine and several studies have shown that side-effects can influence attitudes and subjective well-being negatively (Lacro et al. Reference Lacro, Dunn, Dolder, Leckband and Jeste2002; Lambert et al. Reference Lambert, Conus, Eide, Mass, Karow, Moritz, Golks and Naber2004).
Patients may be reluctant to openly report certain side-effects, especially those of an embarrassing nature (e.g. sexual dysfunction, constipation, nocturnal enuresis). A true reflection of the experience of antipsychotic side-effects is therefore obtained by using a structured assessment tool (Byerly et al. Reference Byerly, Nakonezny, Fisher, Magouirk and Rush2006; Yusufi et al. Reference Yusufi, Mukherjee, Flanagan, Paton, Dunn, Page and Barnes2007; Hynes et al. Reference Hynes, McWilliams, Clarke, Fitzgerald, Feeney, Taylor, Boland and Keating2020). While antipsychotic side-effects are known to impact adherence negatively, it is in fact the distress caused rather than an objective measure of severity that is most relevant (Haddad et al. Reference Haddad, Fleischhacker, Peuskens, Cavallaro, Lean, Morozova, Reynolds, Azorin, Thomas and Möller2014). Eliciting both the frequency of side-effects and the associated distress is therefore essential to obtaining an accurate picture of the patient experience of side-effects. The Glasgow Antipsychotic Side-effects Scale (GASS) is a 22-question self-rating scale for detecting the frequency and distress caused by second generation antipsychotic side-effects (Waddell and Taylor Reference Waddell and Taylor2008). The GASS for clozapine (GASS-C) is a 16 question self-report side-effects scale for those on clozapine (Hynes et al. Reference Hynes, Keating, McWilliams, Madigan, Kinsella, Maidment, Feetam, Drake, Haddad, Gaughran, Taylor and Clarke2015). The use of rating scales over time ensures that the experience of antipsychotic side-effects is captured at regular intervals and facilitates the implementation of interventions to minimise their burden (Lehman et al. Reference Lehman, Lieberman, Dixon, McGlashan, Miller, Perkins and Perkins2004).
While both the GASS and GASS-C are self-report scales, administration by the SMHP facilitates patient involvement in a discussion about their experience of side-effects/associated distress and dialogue on the mechanism of side-effects and management options available (Hynes et al. Reference Hynes, McWilliams, Clarke, Fitzgerald, Feeney, Taylor, Boland and Keating2020). These range from practical tips (chewing sugar-free gum to alleviate dry mouth); to dose manipulation (weighting dose to night time for daytime drowsiness); to the addition of another medicine (laxative for constipation) (Stroup and Grey Reference Stroup and Gray2018). However, those experiencing antipsychotic side-effects have varying priorities when it comes to managing them. Some may opt for no intervention; others may prefer non-pharmacological strategies and some may choose additional medicine to manage side-effects. As such, by meeting face-to-face to assess side-effects and discuss management options, the SMHP can support self-advocacy by recommending patient-centred interventions. Systematic side-effects assessment is offered to all patients following two consecutive weeks of antipsychotic treatment and takes approximately 5 minutes to complete. As the GASS-C was developed and validated by this pharmacy team and the clinical utility of the GASS and GASS-C was assessed by SMHPs at this mental health setting, this step in the medicines optimisation process is research-informed and ensures that those with SMI taking antipsychotics obtain the best possible outcomes from their medicines (Hynes et al. Reference Hynes, Keating, McWilliams, Madigan, Kinsella, Maidment, Feetam, Drake, Haddad, Gaughran, Taylor and Clarke2015, Hynes et al. Reference Hynes, McWilliams, Clarke, Fitzgerald, Feeney, Taylor, Boland and Keating2020).
Individualised patient consultations
While med-rec, the provision of PILs and Medicines Lists and the systematic assessment of antipsychotic side-effects are standardised interventions in the pharmacist-led medicines optimisation process, other patient-facing interventions can arise outside of this. These may include clozapine or lithium consultations which are offered to all patients newly commenced on these medicines during an admission. The consultation includes a discussion around the risks and benefits of these higher risk medicines and monitoring (mandatory for clozapine) associated with their use. Other individualised consultations may be prompted by clinicians or nursing staff in response to a patient-specific query. These individualised consultations may occur at any point throughout the admission and serve to ensure that the patient’s medicines-needs are met as they arise.
Clinician-facing interventions
Physical health monitoring and cardiovascular risk assessment
While the interventions detailed above are ‘visible’ to the patient, there are many others occurring behind the scenes (clinician-facing interventions). The first of these involves the physical health monitoring associated with psychotropic medicines. Antidepressants, antipsychotics and mood stabilisers have side-effects that can lead to physical morbidity and worsening of existing comorbidities including metabolic syndrome, diabetes, dyslipidaemia, cardiovascular disease (CVD), etc. (NICE 2014; Carolan et al. Reference Carolan, Keating, Strawbridge and Ryan2019). These side-effects can worsen quality of and shorten life (NICE 2014; Keating et al. Reference Keating, McWilliams, Schneider, Hynes, Cousins, Strawbridge and Clarke2017). In fact, the majority of deaths in those with SMI are due to preventable physical diseases, in particular, CVD – 2–3 times higher risk of dying from CVD compared with the general population (Sud et al. Reference Sud, Laughton, McAskill, Bradley and Maidment2021).
SMHPs’ roles as medicines experts enable them to support the physical healthcare of those living with SMI by prompting the monitoring of physical health parameters at each weekly review (Table 2) (El-Den et al. Reference El-Den, McMillan, Wheeler, Ng, Roennfeldt and O’Reilly2020). In line with manufacturers’ monitoring recommendations and international guidance, local physical health monitoring guidelines developed by SMHPs and approved by the multidisciplinary Drug and Therapeutics committee (unpublished) are used to ensure those prescribed psychotropic medicines have appropriate physical health screening (Barnes et al. Reference Barnes2011; NICE 2014; Taylor et al. Reference Taylor, Barnes and Young2021). Furthermore, the SMHP can provide advice on how best to manage out of range results in a patient-centred manner for all of those with deranged results (considering concomitant medicines and comorbidities) – ‘don’t just screen, intervene’. Given that the risk of death from CVD is so high in this group, a cardiovascular (CV) risk assessment is a useful intervention that can be carried out by the SMHP using physical health monitoring test results.
Table 2. Physical health monitoring checklist
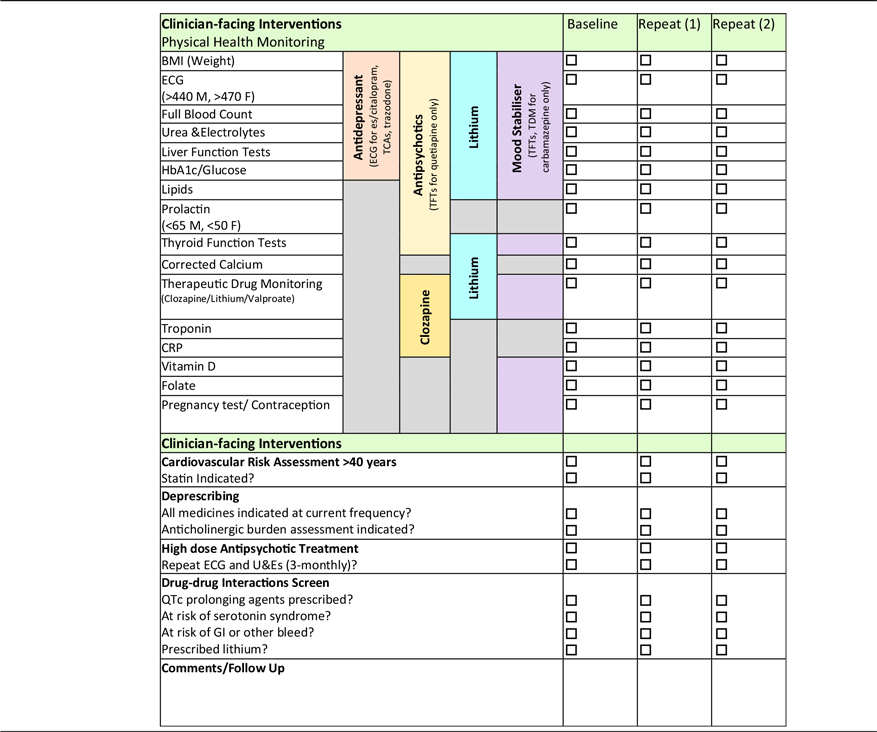
The QRisk3 algorithm calculates a person’s risk of developing a heart attack or stroke over the next 10 years and is used by the SMHP to assess CV risk in those aged > 40 (Hippisley-Cox et al. Reference Hippisley-Cox, Coupland and Brindle2017). By inputting patient-specific demographic and clinical information, a risk score is calculated which indicates whether statin therapy is indicated, i.e., risk score > 10 (NICE 2014). Despite the convincing evidence for increased CV risk in those with SMI prescribed psychotropic medicines (specifically antipsychotics), as well as explicit recommendations provided by guidelines, such screening is often incomplete or inconsistent (Sud et al. Reference Sud, Laughton, McAskill, Bradley and Maidment2021). Thus, a medicines optimisation intervention such as physical health monitoring and CV risk assessment carried out by the SMHP will serve to identify and mitigate the risk of premature mortality in those with SMI.
De-prescribing and anticholinergic burden assessment
While physical health monitoring/management supported by the SMHP may often result in the prescription of a new medicine, “de-prescribing” is equally high on the medicines optimisation agenda. De-prescribing is the process of supervised medication withdrawal, with the goal of managing polypharmacy and improving outcomes (Reeve et al. Reference Reeve, Gnjidic, Long and Hilmer2015). Polypharmacy is commonplace amongst those with SMI. However, as the number of medicine doses per day is inversely related to treatment adherence, eliminating unnecessary medicines and simplifying the medicines regime is critical for a group already at risk of poor adherence (Claxton et al. Reference Claxton, Cramer and Pierce2001).
In an effort to optimise medicines use through de-prescribing, the SMHP clinically reviews all medicines weekly and rationalises the indication and frequency. Medicines that may no longer be indicated, have been consistently declined or are prescribed at a high frequency are brought to the attention of the clinician along with information on how to safely reduce, adjust or discontinue.
Medicines with anticholinergic properties have long been recognised as causing symptoms such as dry eyes, dry mouth, constipation, urinary retention, dizziness, impaired cognition and physical decline (SIGN 2018; King and Rabino 2023). Dry mouth in particular can result in poor oral health including high rates of tooth decay, gum disease and tooth loss – a less recognised health inequality associated with SMI (Tharian et al. Reference Tharian, Patteril and Shah2023; Lancaster University 2022). There may also be an association with falls, increased mortality and CV events (SIGN 2018). Many psychotropic medicines have inherent anticholinergic effects, while others may require the prescription of concomitant anticholinergics to manage extra-pyramidal side-effects. Anticholinergic burden (ACB) scales, such as the ACB calculator can quantify these effects, and provide a practical tool for optimising prescribing (Boustani et al., Reference Boustani, Campbell, Munger, Maidment and Fox2008; King and Rabino 2023). Although not all medicines with anticholinergic properties may individually increase risk of side-effects, when used in combination, effects may accumulate (SIGN 2018). So, by calculating the ACB score, the SMHP can determine the degree of burden the patient is subject to and provide advice to the clinician on how to monitor for and minimise this through de-prescribing.
High dose antipsychotic treatment
By screening and intervening with physical health parameters and ensuring all medicines prescribed are indicated, the SMHP endeavours to achieve the correct balance when it comes to medicine. However, there are some circumstances where this balance is harder to achieve, such is the case for those prescribed high dose antipsychotic treatment (HDAT). HDAT is defined as the prescription of either a single antipsychotic at a dose above the upper limit or two or more antipsychotics concomitantly, that when expressed as a percentage of their respective maximum dose, and combined, result in a cumulative dose of > 100% (excluding clozapine) (Mace and Taylor Reference Mace and Taylor2015). While there is no firm evidence that the prescription of HDAT is more effective than standard doses, there may be some clinical rationale in certain circumstances due to individual patient differences (Mace and Taylor Reference Mace and Taylor2015). In practice, HDAT prescribing rates both locally and internationally sit between 20% and 25% of hospital inpatients (Hynes et al. Reference Hynes, McWilliams, Clarke, Fitzgerald, Feeney, Taylor, Boland and Keating2020). However, as HDAT may be associated with an increased risk of CVD, metabolic syndrome and mortality amongst those with SMI, it must be accompanied by increased physical health monitoring and regular clinical review (Correll et al. Reference Correll, Detraux, De Lepeleire and De Hert2015; Liu et al. Reference Liu, Daumit, Dua, Aquila, Charlson and Cuijpers2017; Das and Yanson Reference Das and Yanson2021). On foot of a prescription of HDAT, the SMHP informs the clinician of the percentage HDAT prescribed along with relevant information on documentation requirements and monitoring criteria to be fulfilled.
Clinically significant drug–drug interactions
There are a number of notable drug–drug interactions specific to the mental health setting. Considering the high rate of physical morbidity and associated polypharmacy in those with SMI, the risk of drug interactions is increased in this population (Khawagi et al. Reference Khawagi, Steinke, Nguyen, Pontefract and Keers2021). Although life-threatening drug–drug interactions are rare, clinically significant interactions impacting response to medicine or resulting in serious adverse drug reactions have been reported and can impact long-term outcomes and patient experience negatively (English et al. Reference English, Dortch, Ereshefsky and Jhee2012). Interactions may occur between two psychotropic medicines or between a psychotropic and a physical health medicine and may occur as a result of the medicines’ pharmacodynamics or pharmacokinetics. In carrying out mental health specific interaction screening, the SMHP ensures that it is safe for all medicines prescribed to be taken concomitantly which is an essential part of medicines optimisation.
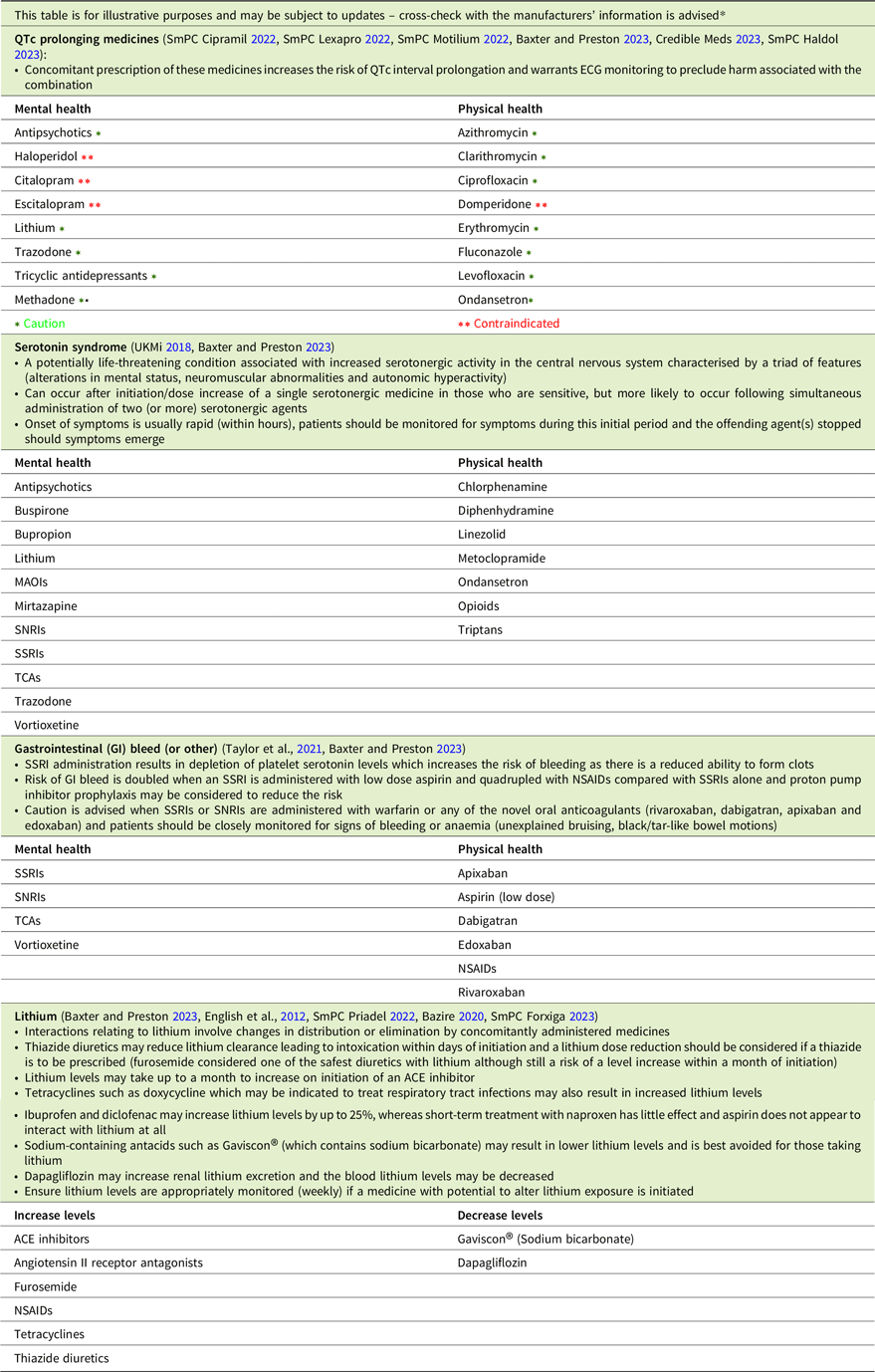
The most clinically significant pharmacodynamic drug–drug interactions include those between medicines that prolong the QTc interval, those that result in serotonin toxicity and those that increase the risk of bleeding, while lithium’s pharmacokinetics account for a range of drug–drug interactions (Table 3). While some medicines that exert these effects are contraindicated when administered concomitantly, others should be prescribed with caution.
Table 3. Drug–drug interactions checklist
Conclusion
In order to ensure those with SMI get the most out of their medicines, pharmacist-led medicines optimisation is essential. By engaging directly with patients to reconcile medicines on admission, provide medicines information in various formats, systematically assess for antipsychotic side-effects and provide one-to-one consultations on request, the SMHP gains an insight into the patient’s relationship with psychotropic medicines. This can inform patient-centred interventions to improve the medicines experience. Additionally, given their knowledge and expertise on both physical and psychotropic medicines, the SMHP can positively influence patient health outcomes through physical health monitoring and management, de-prescribing, high dose antipsychotic monitoring and clinically significant drug–drug interaction screening. Evidence supports the positive impact that SMHPs can have on outcomes, prescribing practices, patient satisfaction and resource use for those in the inpatient mental health setting (Sud et al. Reference Sud, Laughton, McAskill, Bradley and Maidment2021). However, given the limited understanding of what a clinical pharmacy service in a mental health setting looks like, there is a need for a framework that describes evidence-informed interventions. By outlining our medicines optimisation service, this paper may aid other services to adopt pharmacist-led medicines optimisation in their own setting whether it is a hospital or community-based mental health service to improve outcomes for patients with SMI (see SJOG Medicines Optimisation Checklist© in supplementary material).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ipm.2023.46.
Acknowledgements
The authors gratefully acknowledge the contributions of the pharmacy technicians at Saint John of God Hospital – Sinead Askins and Jessica Turner – for their continued support to our medicines optimisation service. We are most grateful to all members of the Drug and Therapeutics Committee at Saint John of God Hospital, chaired by Dr Stephen McWilliams as well as Dr Elizabeth Cummings, Dr Ana DeLeiva, Dr Eric Roche, Dr Brendan Cassidy, Dr Lucy Moran and Dr Ronan Hearne of the Cluain Mhuire Community Mental Health Service for the support in developing and implementing the medicines optimisation service.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
None.
Ethical standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. The authors assert that the local ethics committee has determined that ethical approval for publication of this perspective piece was not required by the local Ethics Committee.





