No CrossRef data available.
Published online by Cambridge University Press: 15 July 2020
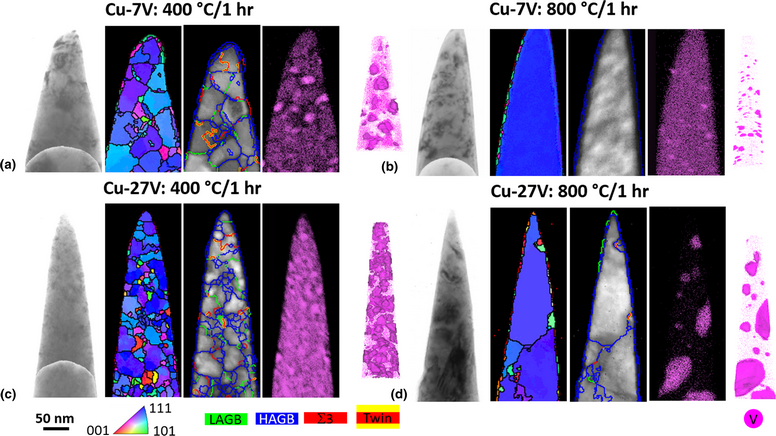
We report the sputter deposition of Cu-7V and Cu-27V (at.%) alloy films in an attempt to yield a “clean” alloy to investigate nanocrystalline stability. Films grown in high vacuum chambers can mitigate processing contaminates which convolute the identification of nanocrystalline stability mechanism(s). The initial films were very clean with carbon and oxygen contents ranging between ~0.01 and 0.38 at.%. Annealing at 400 °C/1 h facilitated the clustering of vanadium at high-angle grain boundary triple junctions. At 800 °C/1 h annealing, the Cu-7V film lost its nanocrystalline grain sizes with the vanadium partitioned to the free surface; the Cu-27V retained its nanocrystalline grains with vanadium clusters in the matrix, but surface solute segregation was present. Though the initial alloy and vacuum annealing retained the low contamination levels sought, the high surface area-to-volume ratio of the film, coupled with high segregation tendencies, enabled this system to phase separate in such a manner that the stability mechanisms that were to be studied were lost at high temperatures. This illustrates obstacles in using thin films to address nanocrystalline stability.