Published online by Cambridge University Press: 16 February 2015
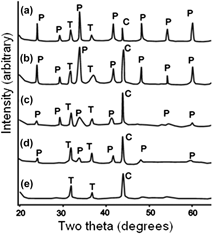
Candidate materials for actinide immobilization are subject to alpha-decay event doses that accumulate to values of more than 1020 alpha-decays per gram (tens displacements per atom, dpa) over the extended periods of geologic disposal. To evaluate the radiation-response of actinide-bearing materials, two experimental techniques have been used to accelerate the damage accumulation process: ion-beam irradiations and 244Cm-doping experiments. Based on modern characterization techniques, such as high-resolution transmission electron microscopy, and experimental results that involve ion-beam irradiation and chemical doping with highly active actinides, crystalline ceramics for the immobilization of actinides can be divided into three groups on the basis of their critical doses, Dc, i.e., the dose required for amorphization at 300 K: (i) low resistance to radiation damage accumulation (Dc ∼ 0.2 dpa) – murataite, Ti-perovskite, Fe-garnet; (ii) resistant (0.4 < Dc < 0.6 dpa) – Al-garnet, Ti–Zr-pyrochlore, Al-perovskite; and (iii) highly resistant (Dc > 0.8 dpa) – Zr-, Zr–Ti-, and Sn-pyrochlores. Phases with low critical temperatures (Tc below 600 K) will not become amorphous in a deep geologic repository, as long as the temperature remains between 300 and 550 K, but rather, they will remain crystalline. Only Zr-rich pyrochlore is fully resistant to radiation damage and will remain crystalline over the entire period of its disposal.
This section of Journal of Materials Research is reserved for papers that are reviews of literature in a given area.
Contributing Editor: William J. Weber