Introduction
The double burden of malnutrition (DBM) is characterised by the coexistence of undernutrition (or micronutrient deficiency) and overweight or diet-related non-communicable diseases (NCDs)(1). DBM is associated with a change in the dietary patterns of the population from a natural diet to a diet with a high content of ultra-processed products(Reference Mozaffarian, Rosenberg and Uauy2,Reference Popkin, Corvalan and Grummer-Strawn3) . It is estimated that more than 20 % of Latin American women have metabolic syndrome (MetS)(Reference Escobedo, Schargrodsky and Champagne4–Reference Rubinstein, Irazola and Calandrelli7), whereas the prevalence of anaemia is 22 % (in women aged 14–49 years)(8). MetS increases the risk of cardiovascular diseases and diabetes(Reference Alberti, Eckel and Grundy9), which are the leading causes of death worldwide(10). On the other hand, anaemia and Fe-deficiency anaemia (IDA) affect physical performance, work productivity(Reference Haas and Brownlie11) and health outcomes during pregnancy and birth(Reference Rahman, Abe and Rahman12). Furthermore, in non-anaemic women, Fe deficiency is associated with anger and fatigue(Reference Sawada, Konomi and Yokoi13), and reduced body Fe is associated with decreased performance on cognitive executive planning function(Reference Blanton, Green and Kretsch14). Even though an individual may be affected by both sides of DBM(1), the association between these sides is still unclear.
Several studies have evaluated the association between overweight and Fe status. Analysis from the Mexican National Nutrition Survey 1999 showed that, in women (18–50 years), obesity is associated with Fe deficiency (OR = 1⋅92; 95 % CI 1⋅23, 3⋅01; P < 0⋅05). Although Fe intake in both obese and non-obese was similar, obese had a lower serum Fe concentration than non-obese (mean (sd) = 62⋅6 (29⋅5) μg/dl v. 72⋅4 (34⋅6) μg/dl; P = 0⋅014)(Reference Cepeda-Lopez, Osendarp and Melse-Boonstra15). Moreover, a recent study including school-aged children from Guangzhou, China, found that obesity was associated with a lower risk of anaemia (adjusted OR = 0⋅553; 95 % CI 0⋅316, 0⋅968; P = 0⋅038) and a higher risk of Fe deficiency without anaemia (ID) (adjusted OR = 1⋅808; 95 % CI 1⋅146, 2⋅853; P = 0⋅011). However, in this work, obesity was not associated with IDA(Reference Zheng, Long and Tan16). The underlying mechanisms for the above-mentioned associations remain unclear. It is postulated that obesity-related inflammation induces overproduction of hepcidin, a key modulator of Fe metabolism, which affects Fe status by decreasing its absorption and increasing intracellular sequestration(Reference Becker, Orozco and Solomons17). Nonetheless, other authors have not found the association of obesity with Fe deficiency without anaemia in Mexican women(Reference Tijerina-Sáenz, Martínez-Garza and Ramírez-López18) or with anaemia in Colombian women(Reference Kordas, Fonseca Centeno and Pachón19).
Considering the latter discrepancy, it is interesting to evaluate the association between MetS and Fe status. A meta-analysis of cross-sectional and prospective studies, comprising data from 78 851 subjects, reported a positive association of high serum ferritin levels (a biomarker of Fe stores) with MetS (OR = 1⋅78; 95 % CI 1⋅60, 1⋅97; heterogeneity P < 0⋅001; I 2 57⋅2 %) and the MetS components. High triacylglycerols (OR = 1⋅96; 95 % CI 1⋅65, 2⋅32; heterogeneity P < 0⋅001; I 2 82⋅8 %) and high glucose levels (OR = 1⋅60; 95 % CI 1⋅40, 1⋅82; heterogeneity P < 0⋅001; I 2 77⋅8 %) were the components most strongly associated with high ferritin(Reference Suárez-Ortegón, Ensaldo-Carrasco and Shi20). Nevertheless, it is important to note that MetS is characterised by chronic low-grade inflammation(Reference Zafar, Khaliq and Ahmad21), which may decrease Fe status. Therefore, we hypothesise that MetS may be positively associated with the different stages of Fe deficiency (ID and IDA) and anaemia. Based on this hypothesis, the objective of the present study was to assess the associations of ID, IDA and anaemia with MetS in Ecuadorian women.
Materials and methods
Data source and sampling
This cross-sectional study was based on data from the 2012 Ecuadorian National Health and Nutrition Survey (ENSANUT-ECU, acronym in Spanish). The survey applied a probabilistic, multistage, population-based sampling design, stratified by clusters (household segments) that included 19 706 households to obtain national and sub-regional representativeness (16 sub-regions). The data collection was performed by trained field workers using standardised procedures, protocols and equipment. Besides, the quality and validity of data were controlled at different levels. Detailed information regarding the data collection procedures, approved by the Ethics Committee of the Universidad San Francisco de Quito is available in the official survey book(Reference Freire, Ramírez-Luzuriaga and Belmont22). The ENSANUT-ECU databases analysed in this study are publicly available(23). The sample for the current analysis comprised 5894 women aged 20–59 years. The inclusion criterion was complete data of variables of interest: age, sex, weight, height, Hb, serum ferritin, waist circumference (WC), High Density Lipoproteins (HDL), Triacylglyceride (TAG), blood pressure, fasting glucose and C-reactive protein (CRP). Furthermore, inflammation status (CRP > 10 mg/l) was the exclusion criterion(24).
Assessment of iron deficiency, iron-deficiency anaemia, anaemia and MetS
In the ENSANUT-ECU 2012, the methods used for the biochemical assessment were sodium lauryl sulphate spectrophotometry (Hb), chemiluminescent immunoassay (ferritin), colourimetric enzymatic-assay (HDL, TAG and glucose) and automated nephelometry (CRP). In the survey, the anthropometric and blood pressure measurements followed established protocols. Weight was measured to the nearest 0⋅1 kg using a digital weighing scale. Height and waist circumference were measured to the nearest 0⋅1 cm with a portable stadiometer and an ergonomic circumference measuring tape, respectively. Blood pressure was measured to the nearest 0⋅5 mmHg with a digital tensiometer. All anthropometric variables and blood pressure were collected twice, with an interval of 5 min. When there was a difference of ±0⋅5 kg in weight, or ±0⋅5 cm in height and waist circumference, or ±5 mmHg in blood pressure, a third measurement was made. The mean value was calculated from the two closest values(Reference Freire, Ramírez-Luzuriaga and Belmont22).
ID was defined as serum ferritin <15 μg/l(25). Anaemia was defined as Hb < 12 g/dl, after adjustment by altitude(26). IDA was considered as the coexistence of serum ferritin <15 μg/l and anaemia(24). The diagnosis of MetS was based on the harmonised definition (2009)(Reference Alberti, Eckel and Grundy9), which requires the presence of at least three of the following criteria: WC ≥ 80 cm; fasting glucose ≥ 100 mg/dl; TAG ≥ 150 mg/dl; HDL <50 mg/dl; systolic blood pressure ≥ 130 and/or diastolic blood pressure ≥ 85 mmHg or antihypertensive drug treatment in a patient with a history of hypertension.
Statistical analysis
The socio-demographic characteristics of the sample were described with frequencies and percentages. Anthropometric variables as height, weight and BMI were described with mean and standard deviation (sd). The remaining continuous variables were described according to their distribution, evaluated by a Kolmogorov–Smirnov test. The prevalence of the stages of Fe deficiency, anaemia and MetS is expressed as frequency, percentage and standard error (se). Prior to the association analysis, the sample was stratified by age decades. The χ 2 test and Fisher's exact test were used to evaluate the possible associations of the stages of Fe deficiency and anaemia with MetS. The prevalence ratio (PR) of ID, IDA and anaemia was estimated considering women without MetS as a reference. The SPSS statistical software package PASW Statistics for Windows version 18.0 (SPSS Inc, Chicago, IL, USA) was used to conduct the analyses.
Ethical considerations
The current research was conducted under the principles of the Helsinki Declaration(27). In Ecuador, the official public data, as the databases analysed in this study(23), are free access according to the Organic Code of Social Economy of Knowledge, Creativity and Innovation(28). Since this is a secondary analysis of anonymised data from a national survey, which were collected following ethical standards(Reference Freire, Ramírez-Luzuriaga and Belmont22), no authorisation is required from a Health Research Ethics Committee.
Results
Most of the participants (93⋅3 %) were 20–49 years, 16 % self-identified as members of an ethnic minority and 44⋅8 % were in the economic status quintiles Q1 or Q2. Table 1 shows other socio-demographic characteristics of the sample. Mean BMI was 27⋅3 (sd 4⋅7; see Table 2), and 67⋅3 % of the women had excess weight (see Table 1). The proportion of women affected by the MetS components was elevated WC 76⋅5 % (se 0⋅6), reduced HDL 68⋅5 % (se 0⋅6), elevated TAG 26⋅9 % (se 0⋅6), elevated blood pressure 15⋅5 % (se 0⋅5) and elevated fasting glucose 14⋅4 % (se 0⋅5). The total prevalence of MetS was 32⋅3 % (se 0⋅6; Table 3). Overall, 81⋅7 % (se 0⋅5) of the women were not affected by the stages of Fe deficiency or anaemia, and 7⋅1 % (se 0⋅3) had IDA (Table 3).
Table 1. Socio-demographic characteristics of participants: Ecuadorian women aged 20–59 years (data from the ENSANUT-ECU 2012)

BMI categories were defined according to WHO: thinness (BMI < 18⋅5 kg/m2), normal (BMI ≥ 18⋅5–24⋅9 kg/m2), overweight (BMI ≥ 25–29⋅9 kg/m2) and obesity (BMI ≥ 30 kg/m2).
a Total = 5894.
b Women with no data n = 3.
Table 2. Anthropometrical, biochemical and clinical biomarkers of participants: Ecuadorian women aged 20–59 years (data from the ENSANUT-ECU 2012)
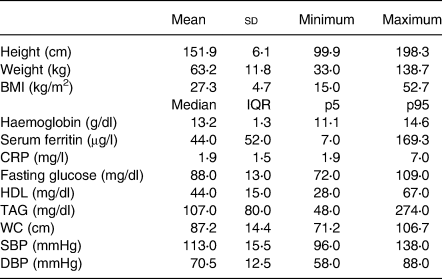
sd, standard deviation; IQR, interquartile range; CRP, C-reactive protein; WC, waist circumference, SBP, systolic blood pressure; DBP, diastolic blood pressure.
For all variables, the significance of the Kolmogorov–Smirnov test was P < 0⋅01.
Table 3. Prevalence of the stages of iron deficiency and anaemia according to metabolic syndrome diagnosis in Ecuadorian women aged 20–59 years (data from the ENSANUT-ECU 2012)

se, standard error; ID, Fe deficiency; IDA, Fe-deficiency anaemia.
ID was defined as serum ferritin <15 μg/l as proposed by WHO(25).
Anaemia was defined as Hb <12 g/dl after adjustment by altitude as proposed by WHO(26).
IDA was defined as the presence of serum ferritin <15 μg/l and anaemia(24).
MetS definition was based on the harmonised definition (2009), with at least three of the following criteria: waist circumference ≥ 80 cm; fasting glucose ≥ 100 mg/dl; TAG ≥ 150 mg/dl; HDL < 50 mg/dl; systolic blood pressure ≥ 130 and/or diastolic blood pressure ≥ 85 mmHg or antihypertensive drug treatment in a patient with a history of hypertension(Reference Alberti, Eckel and Grundy9).
Table 4 reports PR of the stages of Fe deficiency and anaemia (crude and stratified by age) in women with and without MetS. In women less than 50 years, MetS was associated with ID but not with IDA or anaemia. Women of reproductive age (20–49 years) with MetS had a lower prevalence of ID than their counterparts without MetS. PR of ID for these women was (PR (95 % CI; P-value)): 0⋅17 (0⋅06, 0⋅46; P < 0⋅001) (20–29 years); 0⋅69 (0⋅48, 0⋅99; P = 0⋅044) (30–39 years) and 0⋅44 (0⋅29, 0⋅67; P < 0⋅001) (40–49 years). On the other hand, women aged 50–59 years with MetS had a lower prevalence of IDA and anaemia than those without MetS (PR (95 % CI; P-value)): 0⋅12 (0⋅02, 0⋅96; P = 0⋅026) and 0⋅22 (0⋅07, 0⋅64; P = 0⋅002), respectively. No significant association was observed between MetS and ID in women aged 50–59 years (P = 0⋅660).
Table 4. Association of metabolic syndrome with the stages of iron deficiency and anaemia by age, in Ecuadorian women aged 20–59 years (data from the ENSANUT-ECU 2012)

MetS, metabolic syndrome; ID, Fe deficiency; IDA, Fe-deficiency anaemia; PR, crude prevalence ratio; Ref, reference category; 95 % CI, 95% confidence interval.
* Two-tailed P-values for Fisher's exact test, and the remaining two-tailed P-values were assessed by χ 2 test.
Discussion
This work was based on the hypothesis that MetS may be positively associated with the different stages of Fe deficiency (ID and IDA) and anaemia, but the results show the opposite. In Ecuadorian women of reproductive age (20–49 years), MetS is associated with a lower prevalence of ID. Additionally, in women aged 50–59 years, MetS is associated with a lower prevalence of IDA and anaemia. The discrepancies between these two age groups could be attributed to their physiological differences; however, it must be emphasised that the proportion of postmenopausal women is 6⋅8 % of the sample.
Although we did not identify reports evaluating the association between MetS as an independent variable and the different stages of Fe deficiency as dependent variables, in Latin America, this association has been studied in the opposite direction. Leiva et al. found that, in Chilean adults aged 45–65 years, subjects with higher serum ferritin levels had a greater risk of MetS (OR = 3⋅36; 95 % CI 1⋅14, 4⋅20; P < 0⋅001)(Reference Leiva, Mujica and Sepúlveda29). Moreover, they identified that women with MetS had higher levels of Fe parameters than those without MetS: serum Fe (mean (sd) = 122⋅9 (54⋅4) μg/dl v. 108⋅8 (43⋅1) μg/dl; P < 0⋅03), serum ferritin (geometric mean (range) = 53⋅9 (34⋅1, 84⋅8) μg/l v. 27⋅4 (12⋅6, 59⋅5) μg/l; P < 0⋅001) and total body Fe (mean (sd) = 6⋅3 (2⋅3) mg/kg v. 4⋅1 (2⋅8) mg/kg; P < 0⋅001)(Reference Leiva, Mujica and Sepúlveda29). Similarly, in the present paper, higher serum ferritin levels were identified in women with MetS compared with those without MetS (median (interquartile range) = 55⋅0 (66⋅0) μg/l v. 40⋅0 (46⋅0) μg/l; P < 0⋅001) and MetS was associated with a higher prevalence of Fe excess (serum ferritin >150 μg/l(24); PR = 2⋅49; 95 % CI 2⋅05, 3⋅02; P < 0⋅001); results not reported. In this context, we also highlight a study conducted in Brazilian adults reporting associations of high haem Fe intake with increased risk of MetS (OR = 2⋅39; 95 % CI 1⋅10, 5⋅2) and hypertriglyceridaemia (OR = 2⋅51; 95 % CI 1⋅06, 5⋅91)(Reference Dos Santos Vieira, Hermes Sales and Galvão Cesar30).
The causal direction of the association between MetS and Fe nutritional status is unclear. Despite this fact, from biological plausibility, some hypotheses that could explain this association are as follows: the dietary intake of haem Fe increases body Fe stores, which predict the development of type 2 diabetes mellitus(Reference Bao, Rong and Rong31); according to animal model studies, Fe overload causes pancreatic β-cells dysfunction, associated with oxidative stress(Reference Cooksey, Jouihan and Ajioka32) and mitochondrial dysfunction(Reference Jouihan, Cobine and Cooksey33); elevated Fe levels are associated with adipocyte insulin resistance and decreased plasma adiponectin(Reference Wlazlo, van Greevenbroek and Ferreira34); and Fe overload increases muscle glucose uptake despite reduced insulin signalling in animal models(Reference Huang, Simcox and Mitchell35). Finally, elevated serum ferritin does not necessarily reflect high Fe stores(Reference Castiella, Zapata and Zubiaurre36). The levels of this protein are also associated with the chronic low-grade inflammation in MetS(Reference Iwanaga, Sakano and Taketa37). Furthermore, in men and postmenopausal women, MetS is associated with lower serum ferritin levels than those established by WHO as cut-off points for Fe overload(Reference Abril-Ulloa, Flores-Mateo and Solà-Alberich38). It is important to note that the median of serum ferritin (42 μg/l (IQR 48⋅0)) observed in Ecuadorian women (20–49 years) seems to be higher than the median values reported from adult women in developed countries, which ranged from 32 μg/l in women aged 20 to <24 years to 41 μg/l in women aged 48 to <52 years(24). This data could be considered as a success of Fe fortification strategies in Ecuador. However, based on the possible relationship between MetS and Fe status, a detailed follow-up of iron fortification programmes should be encouraged.
On the other hand, according to the new perspectives of nutrition science, to explore the effects of food on diet-related NCDs, it is necessary to move from the nutrient-focused approach to an analysis of the dietary patterns of the population(Reference Mozaffarian, Rosenberg and Uauy2). This new paradigm contrasts with the hegemonic approaches in food and nutrition policies(Reference Mozaffarian, Rosenberg and Uauy2), which have been useful in reducing micronutrient deficiencies(Reference Keats, Neufeld and Garrett39).
It is important to take into account that in Latin American countries, the major determinant of the increase in overweight, obesity and diet-related NCDs is a diet high in saturated fat, sugar and sodium, with high consumption of food of animal origin, refined cereals, and processed and ultra-processed products(Reference Popkin and Reardon40). This diet is replacing the traditional diet and, therefore, the purchase and consumption of natural and minimally processed foods(Reference Martins, Levy and Claro41,Reference Moubarac, Batal and Martins42) , which affects food sovereignty(8) and planetary health(Reference Willett, Rockström and Loken43). All this, in the context of the transformation of the food system into one controlled by international agribusiness, food industries, food retailers and food service companies(Reference Popkin and Reardon40). Thus, greater attention is needed not only to dietary patterns but also to the food system that promotes the consumption of these patterns.
In addition, the mandatorily fortified foods in the region are wheat flour in eighteen countries, maize flour in five countries and milled rice in three countries(44). These cereals are widely consumed(Reference Muthayya, Sugimoto and Montgomery45,Reference Kovalskys, Fisberg and Gómez46) , but contain little fibre and protein(Reference Oghbaei and Prakash47) and high glycaemic index(Reference Atkinson, Foster-Powell and Brand-Miller48). Diets high in glycaemic index may elevate the risk of type-2 diabetes(Reference Livesey, Taylor and Livesey49,Reference Hardy, Garvin and Xu50) and other cardiometabolic diseases(Reference Hardy, Garvin and Xu50). In the region, other mandatorily fortified vehicles with low nutritional quality are vitamin A-fortified sugar in five countries and iodine-fortified salt in eighteen countries(44).
This is relevant in the context of DBM, as observed in Ecuadorian women with the coexistence of MetS (32⋅3 %) and IDA (7⋅1 %) at the population level. DBM requires policies that simultaneously and synergistically address undernutrition, overweight, obesity and diet-related NCDs(1).
An alternative to face all forms of malnutrition is to implement public policies that promote a food system that ensures healthy and sustainable food for the entire population. Such policies should address nutrition from a dietary pattern approach, integrating the cultural, social, environmental, health(51), nutritional and political dimensions of dietary habits. Examples of this are the Brazilian(52) and Uruguayan(53) dietary guidelines that recommend a diet based on natural or minimally processed foods and home-cooked meals with a wide variety of foods of plant origin and moderate amounts of foods of animal origin, limiting the use of culinary ingredients and processed foods, avoiding ultra-processed products, and eating in company and attentively to enjoy food.
Currently, the ENSANUT ECU reported that, in adults, the mean calorie intake is 1822 kcal (women) and 2143 kcal (men), the contribution of each macronutrient to the total energy intake is 61 % for carbohydrate, 13 % for protein and 26 % for fat (12 % for saturated fat)(Reference Freire, Ramírez-Luzuriaga and Belmont22). However, the description of the dietary pattern of this population is not reported.
Moreover, in Ecuador, rice is the main contributor to the daily intake of non-haem Fe and total Fe, providing 24⋅2 and 19⋅4 %, respectively(Reference Freire, Ramírez-Luzuriaga and Belmont22). The above data is interesting because, in the country, the food chosen for mandatory fortification with Fe and other micronutrients is wheat flour(54), while rice fortification is a voluntary measure(Reference Martins, Levy and Claro41), for which no reports or efficacy assessments were found. This situation requires close monitoring, since voluntary fortification initiatives, along with mandatory fortification policies and supplementation programmes (generally targeting children and women of reproductive age), could lead to an excess of micronutrients with negative health consequences(Reference Mejia, Kuo and Beltran-Velazquez55). For this reason, it is required to control voluntary fortification and excessive intake of micronutrients from non-food sources.
Regarding the strengths of the study, it is highlighted that it was conducted on a sample of adult women derived from ENSANUT-ECU 2012, which provides information on the health and nutritional status of the population to contribute to the design of public policies and programmes(Reference Freire, Ramírez-Luzuriaga and Belmont22). Besides, it is an initial approach in the exploration of possible interactions between overnutrition and undernutrition in Ecuadorian women and is relevant for the generation of hypotheses that could be evaluated in further longitudinal studies. Finally, we recognise that the present work presents some limitations: first, the cross-sectional design does not allow causality analysis; second, daily Fe intake, which could mediate in the relationship between Fe nutritional status and MetS, was not assessed. Intake of over-the-counter vitamin C and minerals should also be evaluated since they could play an important role in the present and future health of the Ecuadorian population.
Conclusions
The present study showed that, in Ecuadorian women of reproductive age, MetS is associated with a lower prevalence of Fe deficiency without anaemia. Additionally, the coexistence of MetS and Fe-deficiency anaemia was identified at the population level. These findings require an analysis that transcends the nutrient-focused approach and considers the dietary patterns of the population, as well as the food system that promotes them. This could provide key elements for developing public policies that simultaneously address all forms of malnutrition while promoting food sovereignty.
Acknowledgements
The authors have no acknowledgements to declare.
The main author received financial support from the research group ‘Determinantes Sociales y Económicos de la Situación de Salud y Nutrición’ (M. A. M.-R., grant number 20260002/1033/2018) and from the Sustainability Strategy of the School of Nutrition and Dietetics from the University of Antioquia. D. A. G.-C. and L. I. G.-Z. were supported by the School of Nutrition and Dietetics from the University of Antioquia, and V. A.-U. was supported by the ‘Facultad de Ciencias Médicas’ and the ‘Dirección de Investigación’ from the University of Cuenca in the context of the research project ‘Doble Carga de Malnutrición y Síndrome Metabólico en individuos ecuatorianos entre 10 y 59 años’ (project number CODI 2019-26350). The publication of this paper was supported by the research group ‘Determinantes Sociales y Económicos de la Situación de Salud y Nutrición’ from the University of Antioquia and by the ‘Dirección de Investigación’ from the University of Cuenca.
M. A. M.-R. carried out data management. M. A. M.-R., D. A. G.-C. and L. I. G.-Z. analysed the data. M. A. M.-R., L. I. G.-Z., V. A.-U. and D. A. G.-C. interpreted the results and outlined the discussion section of the paper. M. A. M.-R. and D. A. G.-C. wrote the first draft of the manuscript. All the authors read, edited and approved the final manuscript.
The authors declare that they have no conflicts of interest.






