Introduction
Artificial reefs (ARs) are sunken structures intentionally deployed on the sea floor to serve different objectives such as protecting sensitive habitats, restoring destroyed habitats, enhancing biodiversity, increasing professional and recreational fisheries and creating areas for diving (Fabi et al., Reference Fabi, Scarcella, Spagnolo, Bortone, Charbonnel, Goutayer, Haddad, Lök and Trommelen2015). Decommissioned vessels have been submerged as artificial reefs to enhance fisheries since 1935 (Stone, Reference Stone and D'itri1985) and to support diving tourism by providing new destinations for more than 50 years (Baine, Reference Baine2001). These intentionally or accidentally introduced artificial wreck reefs (AWRs) are important both for recreational activities such as diving and angling (Leeworthy et al., Reference Leeworthy, Maher and Stone2006; Santos et al., Reference Santos, Oliveira and Cúrdia2013) and are also important for environmental recovery and restoration (Baine, Reference Baine2001; Edney, Reference Edney2012; Walker & Schlacher, Reference Walker and Schlacher2014).
The Mediterranean Sea has 4% of the world's wrecks, and 75% of them are known Second World War wrecks (Parliamentary Assembly of the Council of Europe, 2012). The war wrecks are a great challenge to work with, because of their random depths and distances from the coast (Sinopoli et al., Reference Sinopoli, Consoli, Perzia, Romeo and Andaloro2015). There are limited fish community studies on such accidental wrecks, in which ROVs (Consoli et al., Reference Consoli, Martino, Romeo, Sinopoli, Perzia, Canese, Vivona and Andaloro2014; Sinopoli et al., Reference Sinopoli, Consoli, Perzia, Romeo and Andaloro2015) and visual census methods were used (Simon et al., Reference Simon, Joyeux and Pinheiro2013; Sreekanth et al., Reference Sreekanth, Lekshmi and Patil2019). In the Mediterranean Sea, while the fish community studies were mostly conducted with artificial reefs (ARs) such as concrete blocks, pipes and fuel-gas platforms (Charbonnel et al., Reference Charbonnel, Serre, Ruitton, Harmelin and Jensen2002; Fabi et al., Reference Fabi, Grati, Lucchetti and Trovarelli2002; Reference Fabi, Grati, Puletti and Scarcella2004; Lök et al., Reference Lök, Gül, Ulaş, Düzbastılar and Metin2008; Andaloro et al., Reference Andaloro, Castriota, Ferraro, Romeo, Sara and Consoli2011; Gül et al., Reference Gül, Lök, Özgül, Ulaş, Düzbastılar and Metin2011; Scarcella et al., Reference Scarcella, Grati and Fabi2011; Consoli et al., Reference Consoli, Romeo, Ferraro, Sara and Andaloro2013), there was little interest in the artificial wrecks reefs (AWRs) (Simon et al., Reference Simon, Joyeux and Pinheiro2013; Acarlı et al., Reference Acarlı, Kale and Kocabaş2020). Although decommissioned vessels are commonly used worldwide especially for recreational diving tourism as an attraction for divers (Leeworthy et al., Reference Leeworthy, Maher and Stone2006), there are few fish community studies on these intentionally placed wrecks (Arena et al., Reference Arena, Jordan and Spieler2007; Simon et al., Reference Simon, Joyeux and Pinheiro2013; Walker & Schlacher, Reference Walker and Schlacher2014; Paxton et al., Reference Paxton, Newton, Adler, Van Hoeck, Iversen, Taylor, Peterson and Silliman2020; Plumlee et al., Reference Plumlee, Dance, Dance, Rooker, TinHan, Shipley and Wells2020).
Most of the former studies indicated that fish abundance and/or biomass values at ARs are higher than at natural rocky reefs (NRs) and soft sandy bottoms (SBs) (Honório et al., Reference Honório, Ramos and Feitoza2010; Fagundes-Netto et al., Reference Fagundes-Netto, Gaelzer, Coutinho and Zalmon2011; Coelho et al., Reference Coelho, Monteiro, Abecasis, Blot and Gonçalves2012; Simon et al., Reference Simon, Joyeux and Pinheiro2013; Consoli et al., Reference Consoli, Martino, Romeo, Sinopoli, Perzia, Canese, Vivona and Andaloro2014; Ross et al., Reference Ross, Rhode, Viada and Mather2015; Sreekanth et al., Reference Sreekanth, Lekshmi and Patil2019). Scientists have found that fish communities had differences at a regional and local scale affected by currents (Fulton & Bellwood, Reference Fulton and Bellwood2004), depth (Malcolm et al., Reference Malcolm, Jordan and Smith2011), habitat type (Anderson & Millar, Reference Anderson and Millar2004), bottom structure (Grober Dunsmore et al., Reference Grober-Dunsmore, Frazer, Beets, Lindberg, Zwick and Funicelli2008; Schultz et al., Reference Schultz, Malcolm, Bucher and Smith2012) and topographic complexities (Walker et al., Reference Walker, Jordan and Spieler2009). In addition, the roughness of the reefs, reef complexity (Connell & Jones, Reference Connell and Jones1991; Charbonnel et al., Reference Charbonnel, Serre, Ruitton, Harmelin and Jensen2002; Almany, Reference Almany2004; Lingo & Szedlmayer, Reference Lingo and Szedlmayer2006), shelter and food availability (Arena et al., Reference Arena, Jordan and Spieler2007), reef size (Bohnsack et al., Reference Bohnsack, Harper, McClellan and Hulsbeck1994; Bombace et al., Reference Bombace, Fabi, Fiorentini and Speranza1994) and vertical position (height) of the reef (Rilov & Benayahu, Reference Rilov and Benayahu2000; Ajemian et al., Reference Ajemian, Wetz, Shipley-Lozano, Shively and Stunz2015) were reported as factors which affect fish communities in AR areas. While many studies have compared fish communities between ARs and NRs with these factors, few have taken account of how much fish community structure differs by artificial reefs' distance to natural reefs or the existence of NRs near AR areas (Arena et al., Reference Arena, Jordan and Spieler2007; Rosemond et al., Reference Rosemond, Paxton, Lemoine, Fegley and Peterson2018).
Karaburun Peninsula has two artificial wreck reefs which are identical from stem to stern. They were deliberately sunk to the same depth (36.6 m) and onto the fine-grained pebble sediment in the same year (in 2016), aiming to promote the sustainable development of diving tourism. These similar characteristics of the two wrecks supply us with an excellent opportunity to carry out comparative experiments on fish attitudes (assemblages, biomass and species richness) of two AWRs. The main objective of the study was to compare the fish assemblages at the wrecks based on the existing natural reefs in their neighbourhood. We hypothesized that fish abundance of the wreck which is related to the natural reef is higher than that of the more isolated wreck. In this study, we tested the hypothesis that the fish assemblage associated with a wreck can vary according to different complexity level areas of wrecks. We also assessed how much fish communities' feeding guild structure and fish size differ between wrecks. We hypothesized that the abundance of planktivores is higher on the wreck that is closer to NRs because of the vertical relief of the natural reefs. Additionally, we hypothesized that the juvenile fish abundance is higher on the closer wreck which attracts juveniles by providing more shelter than the more isolated wreck. In the study, the frequency of occurrence of fishes at the shipwrecks and whether they were attractive for divers or not were also examined, as ships were sunk to enhance recreational diving tourism. In addition, the abundance of the economic and non-economic fishes was also assessed, because anglers are other users of the AWRs.
Such assessments can help us to understand how the fish communities differ with the existence of NRs near AWRs. Hence, such a comprehensive assessment of the wrecks' value as a tourism and fisheries resource, will be useful to understand how these fish-associated marine structures should be managed by authorities.
Materials and methods
Study site and shipwrecks' characteristics
The study was performed on the two shipwrecks in the Karaburun Peninsula which lies in the north-eastern coast of the Mediterranean Sea. The peninsula is one of the most preferred places for recreational diving (Çulha & Gönül, Reference Çulha and Gönül2019) with rocky reefs and coralligenous habitats. To create new recreational destinations for divers and anglers, two local transportation vessels were intentionally sunk on homogeneous pebble bottom to the coasts of two different islands of the peninsula in 2016 (Figure 1). Recreational divers have been visiting the wrecks for 6 months from May to October. It is possible for the divers to enter the wrecks. These are twin shipwrecks and they have an overall length of 47.6 m, a width of 7.5 m and hull height of 3.2 m. The total height of the wrecks from keel to the top of the main mast is 16 m. Both shipwrecks were located at the same depth (36.6 m). The shipwreck ‘Alaybey’ (ALB) was located on the coast of Büyükada Island and the distance to the nearest natural rocky reef is 100 m. The other wreck ‘9 Eylül’ (DEY) was deployed to the very close Küçükada Island (15 m). The highest point of the natural reefs of Küçükada is almost 13 m. Both natural reefs' surfaces are rugose, with numerous small holes.

Fig. 1. Study site and locations of the artificial wreck reefs.
Data sampling
The wrecks’ fish communities were sampled monthly with diurnal scuba dives between September 2018 and September 2020. Each wreck was surveyed at least 24 times (720 min depth time per diver) during the study period. Four scuba divers used a non-destructive visual census method, called rapid visual counts (RVCs), to sample fish communities at wrecks (Kingsford & Battershill, Reference Kingsford and Battershill2000). Divers carried out censuses at the same time (between 10:00 and 14:00 h) on the same day at both wrecks in order to minimize bias due to daily migrations (Santos et al., Reference Santos, Monteiro and Lasserre2005). Each census could be standardized as 15 min, thanks to both wrecks having the same physical features. To provide diver safety and to collect the most reliable data under difficult working conditions such as the depths (36.6 m) and the large size of the wrecks (overall length: 47.6 m), underwater samplings were conducted by two groups of divers (Figure 2). While two divers surveyed outside (deck, bow, stern, port and starboard sides) of the wrecks (1 m apart from the wreck and bottom), the other two did closed areas (deck-head lounge, engine room, lower-stern lounge, main lounge and bridge). The wreck areas were named according to their complexities: (1) the areas of low complexity were flat parts of the wrecks (wrecks' bottom, decks and sides), (2) the areas of medium complexity were closed areas (main lounge and bridge) and (3) the areas of high complexity were there where pipes, bulkheads and holes (engine room, deck-head and lower-stern lounges) (Figure 2). During the underwater observations, one diver surveyed fishes, and the other recorded the fish community using high-resolution videos (GoPro Hero v. 2018) in order to identify unknown species in each group. While divers swam freely in 15 min, they identified each species to the lowest possible taxonomic level, the abundance of all species and their approximate total lengths to the nearest cm were recorded.
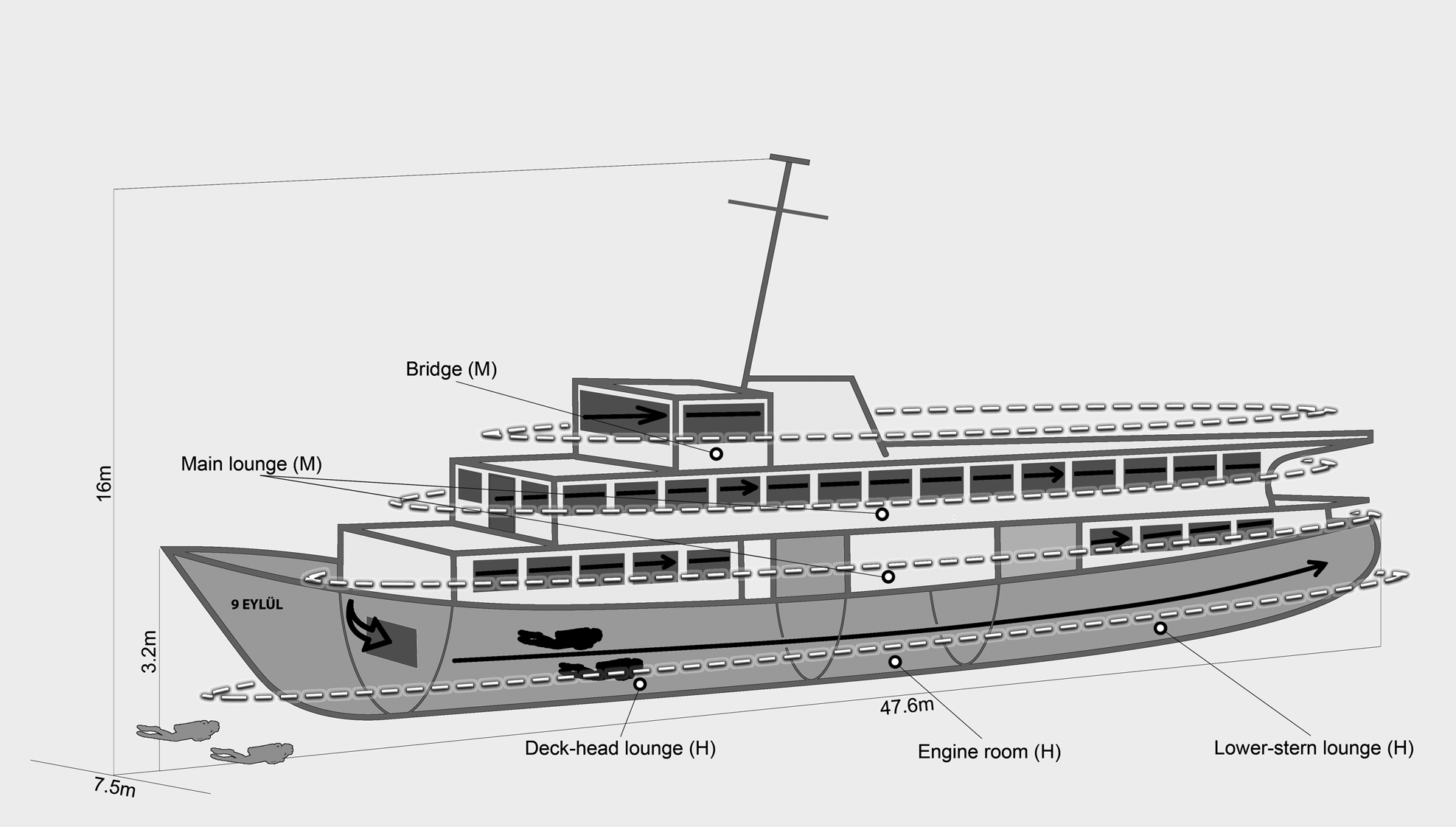
Fig. 2. Visual census survey at the wrecks. Divers shown in grey sampled outside of the wrecks whose path was shown with white dashed line, divers shown in black sampled inside of the wrecks whose path was shown with black line. White dashed lines also show low complexity areas. Letters in brackets state the complexity level of the wreck’ area; M: Medium complexity, H: High complexity.
Data analysis
After recording each species, total length estimates were used for the calculation of biomass by length-weight equations (Harmelin-Vivien et al., Reference Harmelin-Vivien, Harmelin, Chauvet, Duval, Galzin, Lejeune, Barnabé, Blanc, Chevalier, Duclerc and Lasserre1985). When no equation was available, an equation for a mean genus or body shape was applied (Simon et al., Reference Simon, Joyeux and Pinheiro2013). Fishes were categorized into four main groups as follows: (1) different level complexity areas of the wreck (low, medium, high); (2) feeding guilds (carnivores, omnivores, planktivores, herbivores); and (3) juvenile and adult size based on the length of maturity. Five fish species (Boops boops, Diplodus vulgaris, Spondyliosoma cantharus, Apogon imberbis and Chromis chromis) were selected among the species with the highest abundance. These fishes were categorized based on their first maturity lengths (LM) reported in the literature (Bauchot & Hureau, Reference Bauchot, Hureau, Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986; Raventos, Reference Raventos2007; Mouine et al., Reference Mouine, Ktari and Chakroun-Marzouk2011). Since there are no data for LM of C. chromis, field observations were used to determine the maturity length. In our surveys, it was observed that C. chromis that are about 80 mm total length protect and care for the nests, so that was accepted as its LM. In the study, (4) the constancy status of fish was also classified into three groups (accidental, accessory, constant) according to their frequency of occurrence (Dajoz, Reference Dajoz1978). All of the fish species were classified where species recorded in less than 25% of the samples are considered ‘accidental’, species recorded in 25–50% are considered ‘accessory’ and those recorded in more than 50% of the samples are considered ‘constant’.
The economically valuable (fisheries important) species were determined according to the preferences of the local anglers, who stated these fishes as target species to catch in personal interviews with them in Karaburun Peninsula. The commercial importance of each species to recreational and commercial fisheries was also supported by Turkey's National Fisheries Regulations (Anonymous, 2020). Additionally, fishes that attract divers were specified in an interview with Hamdullah Aras, who has been running a diving centre in Karaburun for more than 20 years.
Fish community was examined by means of fish abundance (N), biomass and the number of species (S) using univariate techniques. Shannon–Weaver diversity index (H′), Margalef's Richness index (d) and Pielou's evenness index (J′) were analysed. The homogeneity of variance and normality of abundance data were checked with Levene test and Kolmogorov–Smirnov test (KS), respectively. The fish abundance in different complexity level areas, feeding guilds and body size of selected five species were compared between wrecks using the non-parametric Wicoxon rank sum test (Mann–Whitney U test). The fish abundance differences among locations for each wreck were examined using one-way ANOVA and post-hoc Tamhane (T) comparison of groups' means. Feeding guilds for each wreck's fish abundance were tested by Kruskal–Wallis (KW). Multivariate method was used to detect the similarity of the fish composition among different complexity level areas (low, medium, high) on wreck sites, namely non-metric multidimensional scaling (nMDS) on ranked Bray–Curtis similarities using multi-species abundance data. For abundance data, square-root transformation was used to favour the more abundant species and express the diversity of dominant species (Fowler & Booth, Reference Fowler and Booth2012; Chao et al., Reference Chao, Chiu and Jost2014). SIMPER analysis was used to identify the species percentage contributions to difference among different complexity level areas of wreck sites using Plymouth Routines in Multivariate Ecological Research (PRIMER v7). Spatial variation of the constant and accessory species was determined with both the frequency of species and their abundances and analysed with the Pearson's χ2 test. Both the abundances of the economically important fish and the abundances of the diver attractor fish were compared between wrecks using Mann–Whitney U test. The fish abundance differences between economic and non-economic species for each wreck were also examined using Mann–Whitney U test.
Results
Fish assemblage structure and composition
We recorded 52 fish taxa belonging to 17 families on the Alaybey and 9 Eylül wrecks combined (Table 1). All species were native to the Mediterranean Sea, except Pterois miles. The species was recorded as an invasive species on DEY wreck in March 2020 (Oruç et al., Reference Oruç, Şensurat-Genç, Özgül and Lök2022). An average of 2346 fish individuals per diving count was recorded on the wrecks combined (ALB: 42%, DEY: 58%). The mean abundance of fishes did not differ significantly between wrecks (KSALB P = 0.200, KSDEY P = 0.123; Levene's P = 0.006, t-test P = 0.059). The The 10 most abundant species on wrecks combined were Chromis chromis (2055 ind./dive), Boops boops (1144 ind./dive), Spicara simaris (314 ind./dive), Diplodus vulgaris (294 ind./dive), Spicara maena (276 ind./dive), Spondyliosoma cantharus (188 ind./dive), Apogon imberbis (86 ind./dive), Sardina pilchardus (83 ind./dive), Coris julis (52 ind./dive) and Anthias anthias (44 ind./dive). In terms of spatial distribution at the two wrecks, these 10 species made up 96% and 97% of the total abundance at ALB and DEY, respectively. Chromis chromis was the most abundant species on both wrecks, and it made up 39% at ALB and 47% of total abundance at DEY. Additionally, the mean abundance of C. chromis showed a significant difference between wrecks (KSALB P = 0.119, KSDEY P = 0.104; Levene's P = 0.009, t-test P = 0.020).
Table 1. Abundances, biomass, complexity level of observed location (L: Low, M: Medium, H: High), the status of constancy of fish species (Co: Constant, Acc: Accidental, Acces: Accessory) recorded at the two wrecks
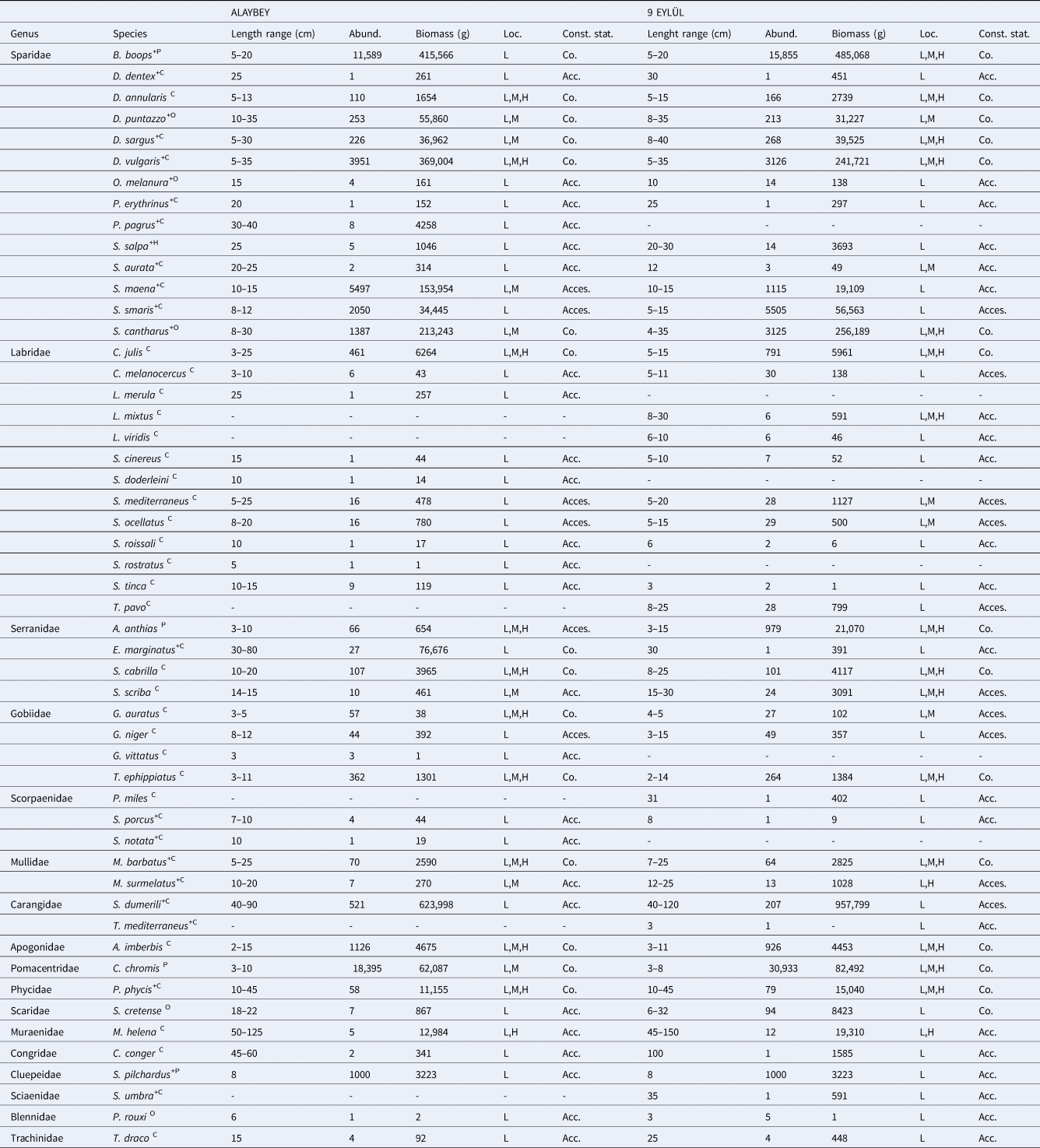
The letters indicated as superscript in the scientific names of the species indicate the feeding guilds (C: carnivores, P: planktivores, H: herbivores, O: omnivores) and the plus sign indicates the economic value of fish.
Total biomass was estimated to be 4375 kilograms fishes on wrecks combined. Even though Alaybey wreck (48%) has less biomass than 9 Eylül wreck (52%), no differences were found in wrecks' biomass (ALB = 87.85 ± 22.56, DEY = 94.82 ± 24.07, U P = 0.635). Seriola dumerili (36%), B. boops (21%), D. vulgaris (14%) and S. cantharus (11%) largely dominated the total biomass. The mean biomass of the most dominant species (S. dumerili) did not significantly differ between ALB and DEY wrecks (ALB = 26.00 ± 22.00, DEY = 39.91 ± 23.60; U P = 0.289).
The five most representative families were Sparidae (14 species), Labridae (13 species), Serranidae (4 species), Gobiidae (4 species) and Scorpaenidae (3 species) on the wrecks combined. Sparidae and Labridae comprised over half of the wrecks’ species pool. Sparidae family had 14 and 13 species on ALB and DEY wrecks, respectively. Pagrus pagrus was only recorded on ALB wreck. Labridae family was represented by 10 species per wreck, however, three species (Labrus merula, Symphodus doderleini and Symphodus rostratus) only were counted on ALB, and another three species (Labrus mixtus, Labrus viridis and Thalassoma pavo) only were seen on DEY wreck. Although Alaybey wreck had slightly greater fish diversity (Shannon–Weaver: 2.68, Margalef's Richness: 4.27) than 9 Eylül wreck (Shannon–Weaver: 2.42, Margalef's Richness: 4.15), there was significantly more mean species richness on DEY than ALB (ALB = 17.00 ± 0.56, DEY = 18.96 ± 0.59, KSALB P = 0.200, KSDEY P = 0.200; Levene's P = 0.948, t-test P = 0.020). SIMPER did not identify important dissimilarity in species composition between the wrecks (average dissimilarity: 14.01%). SIMPER percentages of the top 10 species contributing most to the difference between wrecks are given in Table 2. Some of the MDS plots overlapped in Figure 3 (top left), and the results of the non-metric multidimensional scaling did not show distinct differences (60% dissimilarity) in fish abundances between Alaybey and 9 Eylül wrecks.
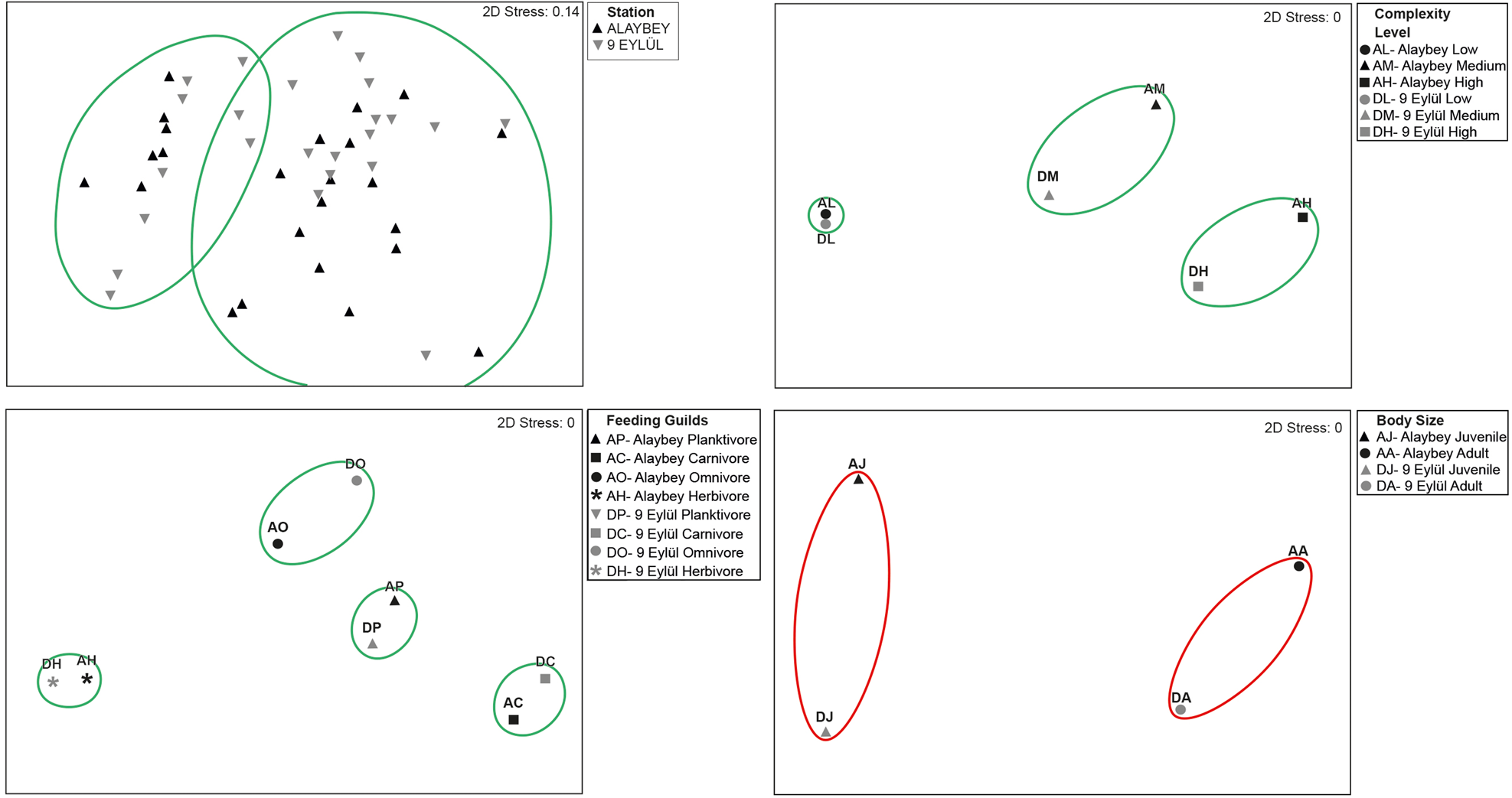
Fig. 3. (A) Non-metric multidimensional scaling (nMDS) plot of Bray–Curtis dissimilarity indices between Alaybey and 9 Eylül wrecks; (B) nMDS of the different level complexity of Alaybey and 9 Eylül wrecks. (C) nMDS of the feeding guilds of fish assemblages on Alaybey and 9 Eylül wrecks. (D) nMDS of the size of fish assemblages on Alaybey and 9 Eylül wrecks. Green and red ellipses indicate 60% and 80% similarities, respectively.
Table 2. Mean abundance (± standard error) and SIMPER percentages contribution of the top 10 fish species contributing to the assemblage differences between Alaybey and 9 Eylül wrecks

Fish abundance at different complexity levels of wrecks
In the survey, fishes were categorized as being in areas of low, medium and high complexity depending on where they were observed at wrecks. The differences were detected among three areas for each wreck's fish abundance (KSALB P = 0.200, Levene's P < 0.001, ANOVA P < 0.001; KSDEY P = 0.178, Levene's P < 0.001, ANOVA P < 0.001) (Table 3). While the highest mean abundance values were recorded in the areas of low complexity (ALB = 1899 ± 194, DEY = 2606 ± 323), the lowest values were obtained in the area of medium complexity of ALB (35 ± 5) and in the area of high complexity of DEY (51 ± 6). There was no significantly greater mean abundance in the area of low complexity of DEY, which is closer to the natural reef, than in the same area of ALB (KSALB P = 0.200, KSDEY P = 0.178; Levene's P = 0.006; t-test P = 0.068).
Table 3. The number of species, mean ± standard error and median of fish abundance between wrecks (* shows significant difference) and among individual wrecks (within a column with differing subscript letters are significantly different, P < 0.05)

SIMPER results showed that there was no substantial dissimilarity in fish abundance between the three areas in both wrecks (average dissimilarity: Low, 15.40%; Medium, 25.86%; High, 23.10%). Spicara maena (22.00%), A. anthias (25.41%) and S. cantharus (21.12%) were the species that contributed most to differences in the areas of low, medium and high complexity, respectively. The nMDS analysis displayed 60% similarity between fish assemblages on wrecks in terms of the areas of different complexity (Figure 3, top right).
At both wrecks, C. chromis and A. imberbis dominated the areas of low and high complexity, respectively. The areas of medium complexity were dominated by D. vulgaris and A. anthias in ALB and DEY, respectively. In the closed and high complexity areas of the wrecks, the number of most fish species decreased, except A. imberbis, followed by Thorogobius ephippiatus.
Fish abundance at feeding guild structure
Fish species were grouped into four feeding guilds as carnivores, omnivores, planktivores and herbivores. The mean fish abundance of feeding guilds is discriminated by wrecks in Table 3. The most abundant trophic guild was planktivores at both wrecks. Planktivores abundance statistically differed between AWRs (KS P = 0.200, Levene's P = 0.004, t-test P = 0.015), DEY (2032 ± 255) having a greater mean planktivores fish abundance than ALB (1294 ± 134). About 59.2% and 63.4% of total abundance of this guild were from only one species, C. chromis at ALB and DEY, respectively. The next highest percentages occurred by B. boops at ALB (37.3%) and DEY (32.5%). Chromis chromis was recorded in all censuses and dominated the planktivore guild. Carnivores, the second most abundant feeding guild at both sites, have more species than other trophic guilds at wrecks. In this guild, 36 and 37 species were recorded at ALB and DEY, respectively. Herbivores were the least abundant trophic guild, which was only represented by Sarpa salpa at ALB (N: 5) and DEY (N: 14). The species was recorded once at ALB (in May) and three times at DEY (in May, July and August).
The abundances of feeding guild groups differed for each wreck (Table 3). The nMDS analysis separates four assemblages at 60% similarity (Figure 3, bottom left). SIMPER analysis revealed that there were 6.12%, 19.23% and 23.00% dissimilarities found on feeding guilds (planktivores, carnivores and omnivores, respectively) between wrecks. Anthias anthias contributed the most to the dissimilarity (47.95%) among planktivore fishes on wrecks. Among carnivore fish species, Spicara maena and Spicara simaris contributed most to the dissimilarity (24.47% and 17.62%, respectively) between the wrecks. Sparisoma cretense contributed most to the dissimilarity (54.27%) among any other omnivores. The only herbivore species, S. salpa was recorded in small numbers on both wrecks, so it did not create any dissimilarity between wrecks.
Fish abundance at body size
To examine differences in fish size between wrecks, the five most abundant species (B. boops, D. vulgaris, S. cantharus, A. imberbis and C. chromis) were classified as juvenile or adult according to their maturity lengths. The majority of these five species were juveniles at both Alaybey (73%) and 9 Eylül (81%). For all species except B. boops at ALB wreck, juveniles have a higher mean abundance than adults on wrecks (Table 3). The juvenile abundances of D. vulgaris, A. imberbis and C. chromis were significantly higher than adults at each wreck. The juveniles of S. cantharus were also in significantly higher abundance than adults at 9 Eylül wreck. The average adult abundance of D. vulgaris and variance of size of C. chromis significantly differed between wrecks (D. vulgaris U P = 0.029; C. chromis t-test P = 0.025).
The nMDS analysis revealed clear differences on wrecks between the fish abundance of juveniles and adults with no overlap (Figure 3, bottom right). SIMPER identified substantial similarities between fish sizes on both wrecks. The juvenile and adult abundances of the five most abundant species had 76.48% average similarity on ALB and 81.96% similarity on DEY. SIMPER also revealed that over 90% similarities in fish sizes (juvenile and adult) were found between wrecks. The juveniles of C. chromis contributed most to the similarity (45.12%), and the adults of B. boops contributed most to the similarity (42.19%).
Constancy of fish species at wrecks
Each wreck assemblage consisted of 46 species, with 15 constant, 6 accessory and 25 accidental species at ALB wreck and with 15 constant, 10 accessory and 21 accidental at DEY wreck. As constant species Diplodus vulgaris, Coris julis, Apogon imberbis and Chromis chromis were recorded on every count on both wrecks. Spatial variation of the most frequent species was characterized by significant differences in the preferences of C. julis (63%) and C. chromis (63%) for DEY wreck, D. vulgaris (56%) and A. imberbis (55%) for ALB wreck. Gobius niger and Symphodus mediterraneus were the top two frequent species among accessory fish, both preferring DEY wreck (53% and 64%, respectively), without significant differences.
Frequency and fish abundance of economic and non-economic fish species
With regard to the fish abundance at ALB and DEY wrecks, 56% and 47% were categorized as economically (fisheries) important fish species, respectively. The majority of these economically valuable fish species were comprised of the Sparidae family at ALB (94%) and DEY (96%). Boops boops was the most abundant fisheries important fish species on each wreck, with 43% and 52% of the economic fish abundance at ALB and DEY wrecks being comprised of the species. Although Seriola dumerili was one of the most targeted fish species by recreational and commercial fishermen in the area, the abundances of this species comprised 2% and 1% of the total abundance at ALB and DEY, respectively. The mean abundances of fisheries important species were compared between Alaybey and 9 Eylül wreck reefs. No difference was found in their abundances between wrecks (ALB = 1111 ± 158, DEY = 1275 ± 244, U P = 0.951).
Frequency and fish abundance of diver attractive fish species
At Alaybey and 9 Eylül wrecks, ~7% of the fish species were categorized as diver attractors. These fish were Epinephelus marginatus, Muraena helena and Seriola dumerili. The abundance of these three diver attractive fish species did not show a significant difference between wrecks. During the study period, while the frequency of E. marginatus, M. helena and S. dumerili accounted for 67%, 21% and 13% at ALB, they were recorded 4%, 38% and 25% at DEY wreck, respectively. During the diving season (from May–October), E. marginatus was seen in 11 counts (total seen 16) at ALB, in once at DEY (total seen 1). Muraena helena was recorded only once (total seen 5) at ALB, and 6 counts at DEY (total seen 9). Seriola dumerili was just seen once (total seen 3) at ALB.
Discussion
The present study demonstrates that the existence of a natural reef nearby did not affect fish abundance and biomass between the wrecks. The similarities in fish abundance and biomass between the two artificial wreck reefs, noted in this study, were supported by the results of the nMDS and SIMPER analysis. Fish abundance and species richness associated with ARs may be related to the degree of isolation (Bohnsack et al., Reference Bohnsack, Johnson, Anderson, Seaman and Sprague1991; Herrera et al., Reference Herrera, Espino, Garrido and Haroun2002; Santos et al., Reference Santos, Oliveira and Cúrdia2013). Herrera et al. (Reference Herrera, Espino, Garrido and Haroun2002) indicated that rocky biotopes near artificial reefs may serve as donor areas and catalyse colonization. Consistent with the authors' findings, in the Karaburun Peninsula, the mean fish abundance, biomass and the number of species (with a significant difference) of DEY wreck near the natural rocky reef were found to be higher than at ALB. However, the DEY that we hypothesized to be more abundant due to its closer proximity to natural reefs, did not show a significant difference from ALB. Similar results were reported by Simon et al. (Reference Simon, Joyeux and Pinheiro2013): total biomass was found to be over four times higher on a century-old shipwreck which is closer to the island than at a decade-old shipwreck which is farther away from the island. The results of the study have shown that the distance between artificial reefs and natural reefs does not present a decisive influence on the fish communities of reefs when considering the age of shipwrecks, as well. Moreover, it is known that structural features of artificial reefs (i.e. complexity) play a greater role than age in determining community structure of ARs (Perkol-Finkel et al., Reference Perkol-Finkel, Shashar and Benayahu2006). In this case, it can be thought that not only the distance to the natural reef and the age of the shipwreck but also the different characteristics of ARs (i.e. complexity, rugosity, vertical profile) and the physico-chemical conditions of the area where the artificial reef is located affect its fish community.
The most abundant families were porgies (Sparidae) and damselfishes (Pomacentridae) for Alaybey and 9 Eylül wreck, respectively. In the Mediterranean Sea, other studies carried out on artificial reefs (concrete blocks, culverts, platforms, wrecks) also reported Sparidae as the most abundant family (Fabi et al., Reference Fabi, Grati, Lucchetti and Trovarelli2002; Santos et al., Reference Santos, Monteiro and Lasserre2005; Gül et al., Reference Gül, Lök, Özgül, Ulaş, Düzbastılar and Metin2011; Özgül et al., Reference Özgül, Lök, Ulaş and Düzbastılar2016; Acarlı et al., Reference Acarlı, Kale and Kocabaş2020). In accordance with other studies from the Mediterranean Sea (Charbonnel et al., Reference Charbonnel, Serre, Ruitton, Harmelin and Jensen2002; Fabi et al., Reference Fabi, Grati, Lucchetti and Trovarelli2002; Relini et al., Reference Relini, Relini, Torchia and Palandri2002; Guidetti, Reference Guidetti2004; Santos et al., Reference Santos, Monteiro and Lasserre2005; Özgül et al., Reference Özgül, Lök, Ulaş and Düzbastılar2016; Acarlı et al., Reference Acarlı, Kale and Kocabaş2020), Sparidae also clearly dominated the fish richness in this study. Shannon–Weaver diversity index had higher values on ALB (2.68) than DEY (2.42), without significant variation between wrecks. H′ were rather higher than obtained values from some studies conducted on ARs in the Mediterranean Sea. While Fabi et al. (Reference Fabi, Grati, Lucchetti and Trovarelli2002, Reference Fabi, Grati, Puletti and Scarcella2004) and Gül et al. (Reference Gül, Lök, Özgül, Ulaş, Düzbastılar and Metin2011) found lower H′ values, Relini et al. (Reference Relini, Relini, Torchia and Palandri2002), Özgül et al. (Reference Özgül, Lök, Ulaş and Düzbastılar2016) and Acarlı et al. (Reference Acarlı, Kale and Kocabaş2020) reported similar H′ values with the present study. Animals across a variety of taxa and levels of the marine food web occur around artificial reefs, ranging from invertebrates to fishes and marine mammals (Paxton et al., Reference Paxton, Steward and Harrison2022). Moreover, high-relief artificial structures have a positive effect on fish diversity by providing additional structures usable by pelagic species (Rilov & Benayahu, Reference Rilov and Benayahu2000; Plumlee et al., Reference Plumlee, Dance, Dance, Rooker, TinHan, Shipley and Wells2020). Wrecks and gas platforms, with a higher relief than concrete structures, extending along the water column, aggregate both benthic and pelagic fishes (Stanley & Wilson, Reference Stanley and Wilson1991; Fabi et al., Reference Fabi, Grati, Lucchetti and Trovarelli2002).
Species richness or abundance have not differed between natural reef areas surrounding artificial wreck reefs and natural reefs with no shipwrecks nearby (Ferro et al., Reference Ferro, Jordan and Spieler2005), which shows that shipwrecks are not attracting fishes away from nearby natural reefs or maybe fish production is occurring on shipwrecks (Arena et al., Reference Arena, Jordan and Spieler2007). On the other hand, the direct (e.g. the exchange of mobile taxa from NRs to Ars; Bohnsack et al., Reference Bohnsack, Harper, McClellan and Hulsbeck1994), and indirect (e.g. loss of herbivores to ARs as a result of food-web alterations; Fowler & Booth, Reference Fowler and Booth2012) effects of artificial reefs were reported on the structure and function of communities on nearby natural reefs. Consistent with these effects, Medeiros et al. (Reference Medeiros, Ferreir, Betancur, Cardoso, Matos and Santos2021) suggested that artificial structures contributed to the degradation of fish diversity in their natural environment. These findings stress the importance of reef site selection. The placement of the artificial reefs should be done with caution in order to conserve and restore the natural reefs and their surrounding aquatic environment (Sheng, Reference Sheng2000), they should not be deployed close to natural reefs or rocky habitats (Medeiros et al., Reference Medeiros, Ferreir, Betancur, Cardoso, Matos and Santos2021). In our study area, the shipwrecks Alaybey (100 m to rocky habitat of Büyükada Island) and 9 Eylül (15 m to rocky habitat of Küçükada Island) were sunk to the locations without any comprehensive analysis to assess potential impacts on the biota of the natural reefs.
Chromis chromis was the most abundant species on each wreck and was also one of the top contributors to similarities seen between the two wrecks, similar to those found on vessel-reefs (herein, artificial wreck reefs) in the Mediterranean (Consoli et al., Reference Consoli, Romeo, Ferraro, Sara and Andaloro2013, Reference Consoli, Martino, Romeo, Sinopoli, Perzia, Canese, Vivona and Andaloro2014; Sinopoli et al., Reference Sinopoli, Consoli, Perzia, Romeo and Andaloro2015; Renzi et al., Reference Renzi, Romeo, Guerranti, Perra, Canese, Consoli, Focardi, Berti, Sprovieri, Gherardi, Salvagio, Giaramita, Esposito, Battaglia, Giacobbe and Andaloro2017; Acarlı et al., Reference Acarlı, Kale and Kocabaş2020). The presence of a physical structure can provide an advantage to fish for protection from predation and can supply feeding on plankton, as well (Sinopoli et al., Reference Sinopoli, Castriota, Vivona, Gristina and Andaloro2012). Wrecks and platforms that show a vertical relief are conducive to the aggregation of C. chromis, facilitating its exposure to plankton, while reducing the risk to be preyed upon (Rilov & Benayahu, Reference Rilov and Benayahu1998; Arena et al., Reference Arena, Jordan and Spieler2007; Renzi et al., Reference Renzi, Romeo, Guerranti, Perra, Canese, Consoli, Focardi, Berti, Sprovieri, Gherardi, Salvagio, Giaramita, Esposito, Battaglia, Giacobbe and Andaloro2017). Our results and underwater observations support these suggestions. At the DEY wreck (closer to the natural reefs) area, the existence of the natural reef creates more current flow than at the ALB area. During one survey at the DEY, divers realized that the wreck was moving away from the natural reef (~3 m). This observation was also supported by the diving operators of the region. The current from the natural reef hits the wreck and creates upcurrent flow and provides planktivore feeding in the upper part of the wreck. Thanks to transporting plankton, the highly abundant C. chromis plays an important active role in transferring energy from the upper to the lower part of the wreck (Pinnegar et al., Reference Pinnegar, Polunin, Videler and De Wijes2007; Sinopoli et al., Reference Sinopoli, Consoli, Perzia, Romeo and Andaloro2015).
DEY wreck had a greater Seriola dumerili biomass without significant difference than ALB. Although the species is not characteristic of the reefs (Santos et al., Reference Santos, Oliveira and Cúrdia2013), it was recorded on artificial wreck reefs and platforms as a transient species (Stanley & Wilson, Reference Stanley and Wilson1991; Fabi et al., Reference Fabi, Grati, Lucchetti and Trovarelli2002, Reference Fabi, Grati, Puletti and Scarcella2004; Arena et al., Reference Arena, Jordan and Spieler2007; Gallaway et al., Reference Gallaway, Raborn, McCain, Beyea, Dufault, Heyman, Putman and Egerton2021). The artificial wreck reefs that provide high vertical relief may support large transient predators (Paxton et al., Reference Paxton, Newton, Adler, Van Hoeck, Iversen, Taylor, Peterson and Silliman2020). In this study, the biomass of S. dumerili on DEY was two and half times higher than ALB wreck; on the other hand, the abundance of this species was higher at ALB. In other words, there were greater lengths of S. dumerili at the wreck, which is close to the natural reef. When considering that the two wrecks are identical and even deployed at the same depths and environmental conditions, it was thought that the reason for the differences in fish demographics (e.g. fish abundance and biomass) was due to the proximity of the DEY wreck to a 15 m high natural reef. Paxton et al. (Reference Paxton, Peterson, Taylor, Adler, Pickering and Silliman2019) found larger fish lengths closer to shipwrecks. Additionally, several previous studies found an inverse relationship between natural reef proximity and reef fish demographics (Bohnsack, Reference Bohnsack1979; Bombace et al., Reference Bombace, Fabi, Fiorentini and Speranza1994), and another indicated proximity to natural reefs did not significantly influence measures of reef fish demographics (Strelcheck et al., Reference Strelcheck, Cowan and Shah2005). To examine how fish abundance and biomass at artificial reefs are affected by changes in the distance to natural reefs requires future extensive research.
Fish species distribution is associated with the shipwreck structure (Renzi et al., Reference Renzi, Romeo, Guerranti, Perra, Canese, Consoli, Focardi, Berti, Sprovieri, Gherardi, Salvagio, Giaramita, Esposito, Battaglia, Giacobbe and Andaloro2017). The three-dimensional arrangement of structures (habitat complexity) has a prominent influence on abundance of fish (Charbonnel et al., Reference Charbonnel, Serre, Ruitton, Harmelin and Jensen2002), fish settlement and recruitment (Cheminee et al., Reference Cheminee, Merigot, Vanderklift and Francour2016) via decreasing predation and/or increasing food availability (Hindell et al., Reference Hindell, Jenkins and Keough2000). In this study, fishes were classified according to their distributed (observed) areas with different levels of complexity of the wrecks as low, medium and high. The comparisons of fish assemblages of these three areas for each wreck support our hypothesis that the fish assemblage associated with a wreck can vary according to the different levels of complexity of the wreck. In terms of fish abundance between the areas (top and bottom), however, Santos et al. (Reference Santos, Monteiro and Lasserre2005) found a difference between artificial reefs (concrete cubic). Contrary to our results, the authors found statistically lower fish abundance at the top of the artificial reefs area in which natural reefs exist, which may be due to the distribution of the fish assemblages in a wider area. Moreover, Sinopoli et al. (Reference Sinopoli, Consoli, Perzia, Romeo and Andaloro2015) reported that the fish assemblage associated with the wreck was not affected by the complexity factor, while our study showed the abundance of most fish species increases with decreasing complexity of the wreck, which may be due to the very active energy transfer provided by planktivores (i.e. C. chromis, the most abundant species) around the artificial wrecks (Bortone et al., Reference Bortone, Cody, Turpin and Bundrick1998). The main lounge and bridge of ALB and DEY wrecks (medium complexity) were dominated by D. vulgaris and A. anthias, respectively. Some authors observed A. anthias as a dominant fish species at the top zones of wrecks in the Mediterranean Sea (Sinopoli et al., Reference Sinopoli, Consoli, Perzia, Romeo and Andaloro2015; Renzi et al., Reference Renzi, Romeo, Guerranti, Perra, Canese, Consoli, Focardi, Berti, Sprovieri, Gherardi, Salvagio, Giaramita, Esposito, Battaglia, Giacobbe and Andaloro2017), however, we counted this species in shade areas of the wrecks. Additionally, dark, closed and shade areas with high complexity were dominated by speleophilic and sciaphilous fishes such as A. imberbis and Thorogobius ephippiatus, for both wrecks. Unlike the above studies which were conducted in the deeper waters of the Mediterranean Sea using ROVs, the depth and method used (UVC) of this study allowed us to penetrate the wrecks, which is probably is reflected in the differences among study results in terms of the dominant fish species.
The DEY wreck which we hypothesized to have higher planktivorous fish abundance than ALB due to its closer proximity to the natural reef, presented an important difference between AWRs. The higher abundances of planktivores on DEY might have resulted from better feeding opportunities in the upper strata of the wreck thanks to the hydrodynamic regime caused by the vertical relief of the natural reefs (Rilov & Benayahu, Reference Rilov and Benayahu2000; Arena et al., Reference Arena, Jordan and Spieler2007). Consistent with our findings, damselfish (C. chromis) represents exclusively planktivorous fish on wrecks in the Mediterranean (Consoli et al., Reference Consoli, Martino, Romeo, Sinopoli, Perzia, Canese, Vivona and Andaloro2014; Sinopoli et al., Reference Sinopoli, Consoli, Perzia, Romeo and Andaloro2015; Renzi et al., Reference Renzi, Romeo, Guerranti, Perra, Canese, Consoli, Focardi, Berti, Sprovieri, Gherardi, Salvagio, Giaramita, Esposito, Battaglia, Giacobbe and Andaloro2017; Acarlı et al., Reference Acarlı, Kale and Kocabaş2020). The swallowtail seaperch (A. anthias), which had a higher abundance on DEY, contributed the most to the dissimilarity among planktivorous fishes between wrecks. These two planktivores provide energy transferal through their faeces (Arena et al., Reference Arena, Jordan and Spieler2007; Renzi et al., Reference Renzi, Romeo, Guerranti, Perra, Canese, Consoli, Focardi, Berti, Sprovieri, Gherardi, Salvagio, Giaramita, Esposito, Battaglia, Giacobbe and Andaloro2017). The nutrients from fish faeces may support both the production of wreck reef benthic communities which are known to strongly influence fish assemblages (Simon et al., Reference Simon, Joyeux and Pinheiro2013) and also may enhance macrofauna colonization on artificial reefs substrate. In a previous study, the macrofauna taxa were reported in the diet of Diplodus spp. in artificial reef areas (Leitão et al., Reference Leitão, Santos and Monteiro2007). AR areas have high importance for these species as feeding areas (Leitão et al., Reference Leitão, Santos, Erzini and Monteiro2008). Our experience supports this suggestion with our underwater observations. Diplodus vulgaris, one of the first colonized species of the artificial reefs (Leitão et al., Reference Leitão, Santos, Erzini and Monteiro2009), was frequently observed feeding from the hard substrates of the wrecks during the study period (see Electronic Supplementary Material 1). The carnivorous species, which include some of the members of genus Diplodus and the other members of Sparidae (Table 1), had the highest abundance following planktivores on both wrecks. The most speciose feeding guild was carnivores with 42 taxa on wrecks combined. It is known that high trophic levels (i.e. carnivores) displayed both high density and biomass in no-take or minimal fishing zones (i.e. marine protected areas) (Guidetti et al., Reference Guidetti, Baiata, Ballesteros, Di Franco, Hereu, Macpherson, Micheli, Pais, Panzalis, Rosenberg, Zabala and Sala2014). Low level of fishing activities, resulting from the diving activities and the risk of fishing gear losses because of the extensions of wrecks, may have protected carnivore fish species at the study site (on wrecks).
In the marine environment, the presence of artificial wreck reefs creates a more complex area for fish by providing shelters with several dimensions. The high vertical relief of wrecks may increase the abundance of juveniles (Rilov & Benayahu, Reference Rilov and Benayahu2002), and artificial wreck reefs and platforms with their complexity structure are used by small prey species for feeding and refuge (Fabi et al., Reference Fabi, Grati, Puletti and Scarcella2004). Additionally, existing natural reefs in the proximity of artificial reefs may also cause higher densities of small fish (herein juvenile) (Santos et al., Reference Santos, Monteiro and Lasserre2005). In this study, the juveniles of five species (B. boops, D. vulgaris, S. cantharus, A. imberbis and C. chromis) composed the majority of the wrecks fish assemblages. The higher juvenile fish abundance could be due to fishing mortality of larger (adult) individuals of commercially important fish (Hall et al., Reference Hall, Herbert and Stafford2021). Moreover, the comparison of juvenile fish assemblages of C. chromis between wrecks supports our hypothesis that the juvenile fish abundance is higher on DEY wreck where there exists a natural reef. On the other side, the mean adult abundances of relatively larger fish (herein, B. boops, D. vulgaris, S. cantharus) among certain fish were found to be higher on ALB wreck. In the Canary Islands, Herrera et al. (Reference Herrera, Espino, Garrido and Haroun2002) reported that artificial reefs near rocky reefs may facilitate colonization, and on the other hand, may also facilitate adult emigration from the simple design of the artificial reefs (i.e. concrete blocks). It is also known that prey on natural reefs encounter predator fish more often than prey on isolated artificial reefs (Connell, Reference Connell1997). Therefore, the juvenile abundances of certain species might have been found higher on DEY wreck than ALB. The juveniles, which can also be called prey, may have come from the natural reefs to the DEY wreck in order to avoid predation pressure. It should also be noted that juveniles and adults of most species have different habitat preferences, so it may be inadequate to assess the impact of only existing natural reefs around artificial reefs on both groups. Age and habitat complexity of the artificial reefs can affect the abundance of juvenile fish by supporting resident predators (Cheminee et al., Reference Cheminee, Merigot, Vanderklift and Francour2016). The harbour character of the artificial reefs makes them both a significant tool for management and a useful area for habitat conservation (Santos et al., Reference Santos, Garcia-Berthou, Agostinho and Latini2011; Fowler & Booth, Reference Fowler and Booth2012).
Constant fish, defined as the persistence of the fish assemblages, are difficult to determine, due to the fact that these depend on not only the place and time of the observation, but also on the experience of the observer (Harmelin, Reference Harmelin1987). In this study, though the areas (artificial wrecks) are highly large and complex, observations give an idea of the status of the fish constancy thanks to the standardized observation method (RVCs, as 15 min) and the efficiency of the divers. The similarity of the two wrecks in terms of constancy status was not surprising, since they are identical and they are even at the same depth (36 m) and on the same type of seafloor (fine-grained pebble). The wrecks were colonized by fundamentally the same species of fish. The proportion of accidental fish species was higher than constant and accessory fish. Muraena helena, C. conger, P. rouxi, G. vittatus, S. porcus and S. notata were recorded as accidental (transient) because their frequency of occurrence was less than 25% (Dajoz, Reference Dajoz1978). However, as noted by Santos et al. (Reference Santos, Monteiro and Lasserre2005) and Whitehead et al. (Reference Whitehead, Bauchot, Hureau, Nielsen and Tortonese1986), these species could have been considered as constant species although they are cryptic and some have nocturnal habits (e.g. M. helena and C. conger). The classification of the residency status of fish can differ, for example, Paxton et al. (Reference Paxton, Newton, Adler, Van Hoeck, Iversen, Taylor, Peterson and Silliman2020) have designated the transient fish as ‘fast-swimming, highly mobile, schooling species, which frequently seen in the water column above or around reefs’ and, the authors classified demersal fish commonly associated with the reef structure or seafloor as ‘resident’. According to the definition (Paxton et al., Reference Paxton, Newton, Adler, Van Hoeck, Iversen, Taylor, Peterson and Silliman2020), B. boops can be designated as transient fish due to its fast-swimming, high mobility and schooling behaviour in the water column, but the species was called ‘constant’ because of the frequency of occurrence at both wrecks in this study. On the other hand, while S. dumerili was called a transient fish in Paxton et al. (Reference Paxton, Newton, Adler, Van Hoeck, Iversen, Taylor, Peterson and Silliman2020), in this study we called it accessory and accidental fish at DEY and ALB wrecks, respectively. The distances of wrecks to natural reefs may affect the fish frequency of occurrence at wrecks. Supporting this hypothesis is not the content of this study, and it will be necessary to examine the species-specific movement behaviour of fish with another study.
AWRs increase fishing success (Stone, Reference Stone and D'itri1985), and they have significantly more fisheries (economically) important species than NRs (Arena et al., Reference Arena, Jordan and Spieler2007). Although we did not compare the commercially important fish assemblages of AWR and NR habitats, the higher abundance of the economic fish appeared to prefer wreck (ALB), which is the farther distance to the natural habitat. Artificial reefs are seen as fishery recruitment areas by recreational anglers and small-scale fisheries (Abecasis et al., Reference Abecasis, Bentes, Lino, Santos and Erzini2013), Diplodus spp. uses them as feeding areas (Leitão et al., Reference Leitão, Santos, Erzini and Monteiro2008). In this study, although the abundances of B. boops, Diplodus puntazzo, D. sargus, D. vulgaris, S. maena, S. smaris and S. cantharus (members of Sparidae family) are considerably high around both wrecks, they are not exploited by professional and sporting fishers because of the wrecks' inconvenient fishing conditions (i.e. ropes, mast, sharp parts). During underwater surveys, a good deal of hooks and several anchors were recorded which were stuck on the wrecks. In the interview with the local fishermen, they stated that ‘the gain we make from the fish caught on the shipwrecks is not enough to cover what we will lose’. On the other hand, the two artificial wreck reefs were surrounded by purse seine fishermen in November 2021. We and the dive operators of the region thought that the target species of the fishermen were S. dumerili. The nets were stuck on the wrecks as ghost fishing nets for a week (see Electronic Supplementary Material 2) before being removed by us and other divers from the region. The fact that the wrecks host many divers during the diving season (from May–October) acts as a natural protection measurement that prevents fisheries, but the lack of the legal measurement during the closed diving season (from November–April) can cause such losses and damage for both the marine environment and also fishers and divers.
Large and unusual shaped marine fishes, such as groupers (Giglio et al., Reference Giglio, Alves, Gerhardinger, Grecci, Daros and Bertoncini2014), eels and morays (Tribot et al., Reference Tribot, Carabeu, Deter, Claverie, Villéger and Mouquet2018) are among the attractions for diving tourism. In this study, three diver attractive fish species have been determined around the wrecks. These are M. helena, E. marginatus and S. dumerili. The only invasive fish at the wrecks area, Pterois miles, is also attractive for divers with its aesthetic and beautiful appearance (Jimenez et al., Reference Jimenez, Andreou, Hadjioannou, Petrou, Alhaija and Patsalou2017; Tribot et al., Reference Tribot, Carabeu, Deter, Claverie, Villéger and Mouquet2018). After the northernmost record of the species at DEY wreck (Oruç et al., Reference Oruç, Şensurat-Genç, Özgül and Lök2022), with the abundance of P. miles having gradually increased, it is considered that lionfish may be one of the most preferred fish to see for divers. Nudibranchs, which attracted especially experienced divers with their colours (Cater, Reference Cater, Garrod and Goosling2008), were also among the attractive focal species of the wrecks. Moreover, eggs of Loligo vulgaris (European squid) were among the marine creatures that attract divers to take photographs or just to see them around wrecks. Even though finding squid eggs in the wild seems to be very challenging (Cabanellas-Reboredo et al., Reference Cabanellas-Reboredo, Calvo-Manazza, Palmer, Hernández-Urcera, Garci, González, Guerra, Morales and Nin2014), they are in plain sight on the masts and ropes of ALB and DEY wrecks. Additionally, European spiny lobster (Palinurus elephas) has a diver attractive feature via its appearance with long antennas. The lobster only was recorded on the sediment of the ALB wreck area (see Electronic Supplementary Material 3). As well as species that attract divers, some fish attributes (abundance, variety, large and unusual fishes) would increase the attractiveness of a diving area (Williams & Polunin, Reference Williams and Polunin2000). To contribute to the attractiveness of an area for divers, the fish attributes should be accepted as the most important elements and with management measures would enhance diving tourism.
Put as a whole, this study shows that although deploying artificial wreck reefs near natural rocky reefs catalyses fish colonization, it does not present an exact influence on the fish communities of AWRs. It is known that sinking ships contribute to the degradation of living creatures at natural reefs (Sheng, Reference Sheng2000), for that reason alone, while choosing the deployment area, the benefit of the marine ecosystem should be the most important criterion to be taken. When creating shipwrecks to serve recreational divers and anglers, the chosen deployment area should work for both marine environment and recreational users. Specific to the study area (Karaburun Peninsula), 9 Eylül wreck, which is close to the natural rocky reef, meets divers' expectations providing both a wreck diving experience and rocky reef diving together. However, we do not yet know the effect of thiw wreck on the fish community on the nearby natural reef.
Conclusions
Artificial wreck reefs seem a useful tool as a recreational resource for divers (Şensurat-Genç et al., Reference Şensurat-Genç, Shashar, Özsüer and Özgül2022). In addition to the wrecks' own charm, ample marine life with fish abundance and diversity that gather around wrecks add extra attraction to them. However, a solely socio-economic assessment of shipwrecks is not enough to manage the marine resource, it needs to be assessed from the point of both divers and ecosystem together. To minimize the potential negative impact on the marine ecosystem, changes in the presence/absence and movement pattern of organisms are needed to investigate the natural reefs around the AWRs site before and after deployment.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315422001011.
Acknowledgements
The authors would like to thank diving instructor Mr Hamdullah ARAS for sharing his historical knowledge about the study area. We also would like to thank Dr Semih ENGİN and Mr. Bülent KILINÇ for underwater photographs.
Author contributions
Tuğçe Şensurat-Genç: Conceptualization, Formal analysis, Investigation, Methodology, Writing orijinal draft; Altan Lök: Conceptualization, Investigation, Methodology; Aytaç Özgül: Conceptualization, Investigation, Methodology; Adnan Çağlar Oruç: Investigation, Methodology, Figures and Tables Editing.
Financial support
This work was founded by the Scientific and Technological Research Council of Turkey [Grant no. 117Y033].
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.








