Introduction
Determining the capacity of organisms to acclimate to changing conditions is key to understanding how they might respond to future environmental change (Foo & Byrne, Reference Foo and Byrne2016). Acclimation to specific environmental conditions often occurs through developmental plasticity, which optimizes a phenotype–environment match during the early stages of an organism's development (Dewitt et al., Reference Dewitt, Sih and Wilson1998; Agrawal, Reference Agrawal2001). However, environmental conditions can change, and an ability to respond rapidly to temporal environmental variability is also essential for survival, especially for sessile organisms unable to move to more favourable conditions (Bradshaw, Reference Bradshaw1965; Barua & Heckathorn, Reference Barua and Heckathorn2004). Therefore, sessile organisms have evolved strategies to maximize their ability to feed (Arkema, Reference Arkema2009), compete with other species (Chadwick & Morrow, Reference Chadwick, Morrow, Dubinsky and Stambler2010) and reproduce (Sarà, Reference Sarà1984).
Marine sponges (Phylum: Porifera) are sessile suspension feeders that are an important component of the benthic fauna throughout temperate, tropical and polar habitats, fulfilling a wide range of ecological roles (Diaz & Rutzler, Reference Diaz and Rutzler2001; Bell, Reference Bell2008). Importantly, sponges exhibit high levels of morphological variation, often due to differences in local environmental conditions, which is thought to facilitate their survival in a range of different environments (Gaino et al., Reference Gaino, Manconi and Pronzato1995). Sponges have been shown to exhibit plasticity in many ways, from their gross morphology (e.g. Lopez-Legentil et al., Reference Lopez-Legentil, Erwin, Henkel, Loh and Pawlik2010) to their metabolic physiology (e.g. Morley et al., Reference Morley, Berman, Barnes, de Juan, Downey and Peck2016), and it is important to know which, if any, environmental cues explain this variation. For example, wave action exposure (Palumbi, Reference Palumbi1986), light intensity (Becerro et al., Reference Becerro, Uriz and Turon1994; Uriz et al., Reference Uriz, Turon, Becerro, Galera and Lozano1995), temperature (Schönberg & Barthel, Reference Schönberg and Barthel1997; Mercurio et al., Reference Mercurio, Corriero, Scalera Liaci and Gaino2000; Subagio et al., Reference Subagio, Setiawan, Hariyanto and Irawan2017), sedimentation level (Manconi & Pronzato, Reference Manconi and Pronzato1991; McDonald et al., Reference McDonald, Hooper and McGuinness2002) and other environmental factors (Hadi et al., Reference Hadi, Hadiyanto, Budianto and Niu2015) have all been correlated with various sponge features including morphology, spicule number and levels of spongin. These factors are so closely tied it has even been recently suggested that sponge functional morphologies could be used to inform about the specific environmental conditions in which they are living (Schönberg, Reference Schönberg2021). These differences in morphology can be extreme, and individuals within the same species have been found to vary greatly depending on their local environmental conditions (e.g. Bell et al., Reference Bell, Barnes and Turner2002). However, most studies examining variability in sponge morphology have focused on spatial variation (e.g. Bell & Barnes, Reference Bell and Barnes2000; Bell et al., Reference Bell, Barnes and Turner2002; Farnham & Bell, Reference Farnham and Bell2018; Meyer et al., Reference Meyer, Roberts, Rapp and Davies2019) and there is a paucity of studies looking at temporal changes in sponge morphology.
Sponge species found in the class Demospongiae typically have skeletons made of silica in the form of spicules (Müller et al., Reference Müller, Li, Schröder, Qiao and Wang2007), which provide important structural support. The proportion of spicules (inorganic material) vs live tissue (organic material) is thought to influence the structural strength and toughness of the sponge. Palumbi (Reference Palumbi1984) showed that the strength and stiffness of sponges were influenced by their environment, with sponges living in higher wave energy environments having greater inorganic content than those living in lower wave energy environments. As well as this, levels of inorganic material, including changes in spicule size and number, have been shown to be directly influenced by temperature (Mercurio et al., Reference Mercurio, Corriero, Scalera Liaci and Gaino2000; Subagio et al., Reference Subagio, Setiawan, Hariyanto and Irawan2017). The proportion of inorganic content tends to be greater at colder temperatures, though not necessarily due to increases in spicule size and number and potentially due to a decrease in organic material (Schönberg & Barthel, Reference Schönberg and Barthel1997). It is not only local environmental conditions that can influence the organic vs inorganic content of sponges. Gametogenesis may result in an increase in organic material within a sponge as reproductive elements develop, while gametic or larval release would cause a sudden drop in the ratio between organic/inorganic material. The reproductive timing of sponges is also known to be influenced by environmental conditions (e.g. Witte et al., Reference Witte, Barthel and Tendal1994; Xue et al., Reference Xue, Zhang and Zhang2009; Bautista-Guerrero et al., Reference Bautista-Guerrero, Carballo and Maldonado2010; Zarrouk et al., Reference Zarrouk, Ereskovsky, Mustapha, Abed and Pérez2013), potentially making it difficult to distinguish between reproductive and environmentally associated changes in organic content. Sponge organic content can change with food availability, as in low food environments sponges may need to use organic storage compounds to meet metabolic demand; this would increase the proportion of inorganic material in the sponge.
In this study we explored the seasonal morphological acclimation ability of two sponge species. We measured temporal variability in the organic and inorganic content of two species of demosponges found on the west Wales coast, Halichondria panicea (Pallas, 1766) and Hymeniacidon perlevis (Montagu, 1818). We hypothesized that inorganic material would be higher in the winter months due to an increase in wave action (after Palumbi, Reference Palumbi1984), and decrease in temperature (Schönberg & Barthel, Reference Schönberg and Barthel1997; Mercurio et al., Reference Mercurio, Corriero, Scalera Liaci and Gaino2000; Subagio et al., Reference Subagio, Setiawan, Hariyanto and Irawan2017), and that as reproductive timings of H. perlevis and H. panicea have been shown to depend on environmental conditions, including temperature (Witte et al., Reference Witte, Barthel and Tendal1994 and Xue et al., Reference Xue, Zhang and Zhang2009, respectively), a sudden drop in organic content is likely to occur at some point in the year consistent with their previously described reproductive timings.
Materials and methods
This study was conducted at two rocky shore sites on the west coast of Wales, north of Aberystwyth. The first site was located at the south end of Clarach beach (52.43211 −4.08016) and the second site was located ~6 km north of the first site (52.47976 −4.05238) at the south end of Borth beach. Both sites are moderately exposed intertidal rocky areas with typical north-east Atlantic zonation patterns (Lewis, Reference Lewis1964). The area consists of reef platforms (90–100% bedrock), with many small gulleys and rockpools that support abundant sponge populations on the very low shore. The sponges Halichondria panicea and Hymeniacidon perlevis are abundant on the lower shore, although H. perlevis generally extends further up the shore than H. panicea.
Hymeniacidon perlevis, family Halichondriidae (Montagu 1818), is typically orange to red and varies from encrusting to massive in shape (Gaino et al., Reference Gaino, Frine and Giuseppe2010). It occurs in the littoral zone of the UK coastline all year round (Stone, Reference Stone1970a). Hymeniacidon perlevis is viviparous (Stone, Reference Stone1970b) with ova being fertilized in the sponge, where they develop into larvae that are eventually released (Xue et al., Reference Xue, Zhang and Zhang2009). The reproductive season of H. perlevis occurs from April to August in temperate seas (spring/summer in the northern hemisphere) (Topsent, Reference Topsent1911; Levi, Reference Levi1956; Wapstra & van Soest, Reference Wapstra, van Soest, Vacelet and Boury-Esnault1987; Gaino et al., Reference Gaino, Frine and Giuseppe2010), although the exact timing depends on various environmental conditions (Xue et al., Reference Xue, Zhang and Zhang2009). This species also reproduces by fragmentation (Stone, Reference Stone1970b), potentially when conditions for larval settlement are not suitable. Halichondria panicea, family Halichondriidae (Pallas, 1766) is another intertidal sponge, varying from encrusting to massive in shape (Vethaak et al., Reference Vethaak, Cronie and Van Soest1982) and is widely found in the shallow coastal waters of temperate regions (Witte et al., Reference Witte, Barthel and Tendal1994). Like H. perlevis, H. panicea is viviparous (Amano, Reference Amano1986), with larval release occurring from May to October in temperate seas (spring/summer in the northern hemisphere) (Barthel, Reference Barthel1986; Witte et al., Reference Witte, Barthel and Tendal1994; Gerasimova & Ereskovsky, Reference Gerasimova, Ereskovsky, Custódi, Lôbo-Hajdu, Hajdu and Muricy2007).
Thirty samples were collected of each species every month from both sites between July 2004 and February 2005, and March 2004 and February 2005 for H. perlevis and H. panicea, respectively. Each sponge sampled was at least 1 m apart from each other to reduce the potential for sampling clones. While some identical genotypes (patches) may have been sampled in different months, this is only likely to account for a small number of the total samples collected since the total population sizes across the shores were in the 1000s. Approximately 5 cm2 of each sponge was collected, and in no cases were complete sponges collected. Samples were preserved in 90% ethanol, which was then poured off before the samples were washed in fresh water and dabbed dry before drying. Any internal and external contaminants (e.g. shell material) were removed from the samples before being transferred to a pre-weighed crucible (crucibles were labelled and then washed, rinsed in distilled H2O and dried at 60°C for 2 h, before being weighed). Crucibles containing samples were then transferred to an oven and dried at 60°C for at least 4 h. Crucibles containing samples were then weighed together, before subtracting the weight of the crucible to obtain dry weight. Crucibles containing samples were then placed in a furnace and combusted at 400°C for 6 h. These were left in a desiccator at room temperature to cool, then weighed again before subtracting the weight of the crucible to obtain ash weight. Ash weight gave the inorganic (spicule) content of the sample, and this was removed from the sample dry weight to give the ash free dry weight, or organic content of the sample. These were then used to calculate percentage organic and inorganic content for each sample.
Wave height data and sea surface temperature data were taken from the Irish Data Buoy Network (2004) from weather buoy M5 in the Irish Sea off the coast of Pembrokeshire. Mean daily wave height and mean daily sea surface temperature for the sampling months were averaged to give mean monthly wave height and mean sea surface temperature from March 2004 to February 2005.
Normality of the inorganic weight was visually assessed using quantile-quantile plots. Then, two-way analysis of variance tests (ANOVAs) were performed, including interaction effects, to determine the effect of month and site on mean percentage organic and inorganic content of H. perlevis and H. panicea and to determine whether inorganic content changed over time. Non-significant interaction effects were removed from the model. If the results of the ANOVA were significant (P < 0.05), a Tukey's post-hoc test was performed to disentangle differences among groups. We tested for collinearity of temperature and height with an ANOVA. They appeared to only exhibit a weak negative correlation (R 2 = 0.54) so both temperature and wave height were analysed in the final model. To determine the effects of wave height and temperature on the percentage of inorganic content of H. perlevis and H. panicea, a multiple linear regression model was fitted for each species. Models had inorganic content as the outcome variable, and wave height, temperature, and the interaction between these two as predictors. Non-significant effects were removed from the models sequentially. Interaction effects, if present, were then analysed to find estimated marginal means at different wave heights/temperatures. As environmental data were taken from the same weather buoy for both sites, differences were not analysed. All data were analysed using the software R Studio (RStudio Team, 2016).
Results
Halichondria panicea
Mean inorganic content of H. panicea differed significantly between sites (ANOVA, F(1, 433) = 11.782, P < 0.01) and months (ANOVA, F(7, 433) = 70.973, P < 0.05). Interaction effects were also found to be significant (ANOVA, F(6, 433) = 3.072, P < 0.01), so they were included in the final model. Post hoc comparisons using the Tukey Honestly Significant Difference (HSD) test indicated that the mean inorganic content of H. panicea was significantly higher at Borth compared with Clarach in August and September (P < 0.01) (Figure 1A).
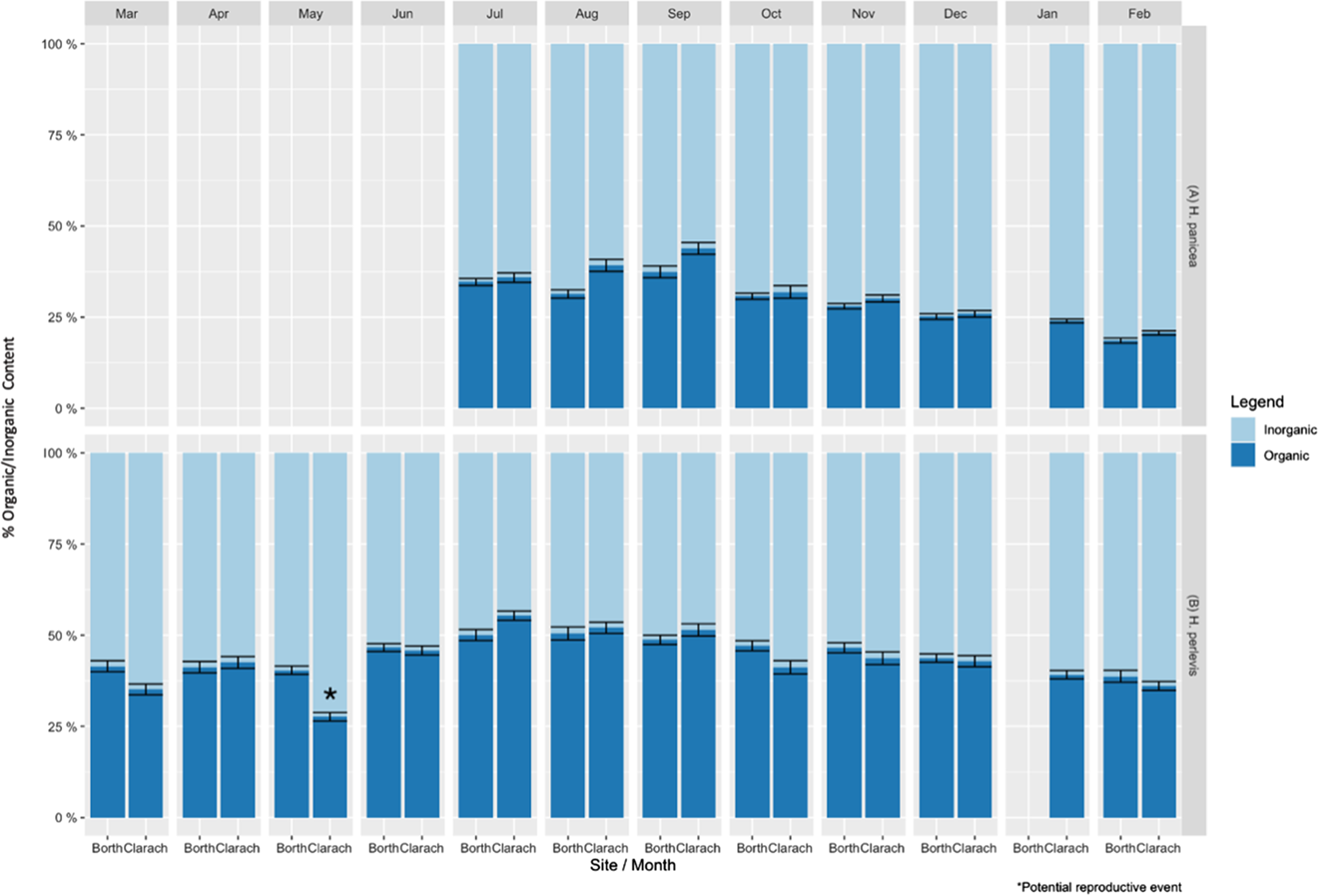
Fig. 1. Mean percentage organic and inorganic content ± standard error of (A) Halichondria panicea and (B) Hymeniacidon perlevis at Borth and Clarach for each month surveyed. * marks potential reproductive event.
Post hoc comparisons using the Tukey HSD test indicated that at Clarach H. panicea had significantly lower inorganic content during September than during all other months (P < 0.05). Levels of inorganic content then generally increased from September to February, where H. panicea had significantly higher mean inorganic content than all other months except December and January (P < 0.05). At Borth, September had significantly lower mean inorganic content than all months except July (P < 0.05). Inorganic content then generally increased until February, when it was higher than all other months (P < 0.05) (Figure 1A).
Hymeniacidon perlevis
Mean inorganic content of H. perlevis differed significantly between sites (ANOVA, F(1, 660) = 13.881, P < 0.01) and months (ANOVA, F(11, 660) = 31.486, P < 0.01). Interaction effects between site and month were also significant (ANOVA, F(10, 660) = 6.086, P < 0.01) so they were included in the final model. Post hoc comparisons using the Tukey HSD test indicated that mean inorganic content of H. perlevis was significantly higher at Clarach than Borth in May (P < 0.01). No other months had significantly different inorganic content between sites (P > 0.05) (Figure 1B).
Post hoc comparisons using the Tukey HSD test indicated that at Clarach the highest mean inorganic content of H. perlevis was in May, significantly higher than during any other month (P < 0.05). The second highest level of inorganic content was found in March (P < 0.05), which then decreased through to July, which had the lowest inorganic content, significantly lower than all months except August and September (P < 0.05). Inorganic content then increased again through to February. There was also a sudden increase in the level of inorganic content from April to May at Clarach, which did not follow the general month to month trend, potentially due to a sudden drop in organic content levels which may indicate a reproductive event (e.g. spawning, Figure 1B).
Post hoc comparisons using the Tukey HSD test indicated that at Borth H. perlevis had the highest inorganic content during February, significantly higher than all months except December and March (P < 0.05). Inorganic content then generally fell until July and August, which had significantly lower levels than all months except May, June and September (P < 0.05). No other differences between months were found to be significant (P > 0.05) (Figure 1B).
Wave height and temperature
Temperature had a significant effect on inorganic content (P < 0.01) (F(1446) = 354, R 2 = 0.44) of H. panicea. Specifically, for every 1 unit increase in temperature, the inorganic content of H. panicea decreased by 2.35% (Figure 2). Interaction effects between temperature and wave height were not significant. Temperature and wave height also had a significant correlation with the inorganic content of H. perlevis (P < 0.01), and their interaction effect was also significant (P < 0.01), so was kept in the final model, which explained 24% of the variation in inorganic content (F(3679) = 74.14, R 2 = 0.24). Further analysis of interaction effects found that at mean wave height (2.4 m) and low temperatures (mean – 1 SD = 10.1°C), inorganic content would be 61.9% (95% CI: 60.8–63.0%). However, at mean wave height (2.4 m) and high temperatures (mean + 1 SD = 15.3°C), inorganic content would be 51.8% (95% CI: 50.3–53.3%). These differences show the effects of wave height on H. panicea to be strongest at low temperatures (Figure 3).
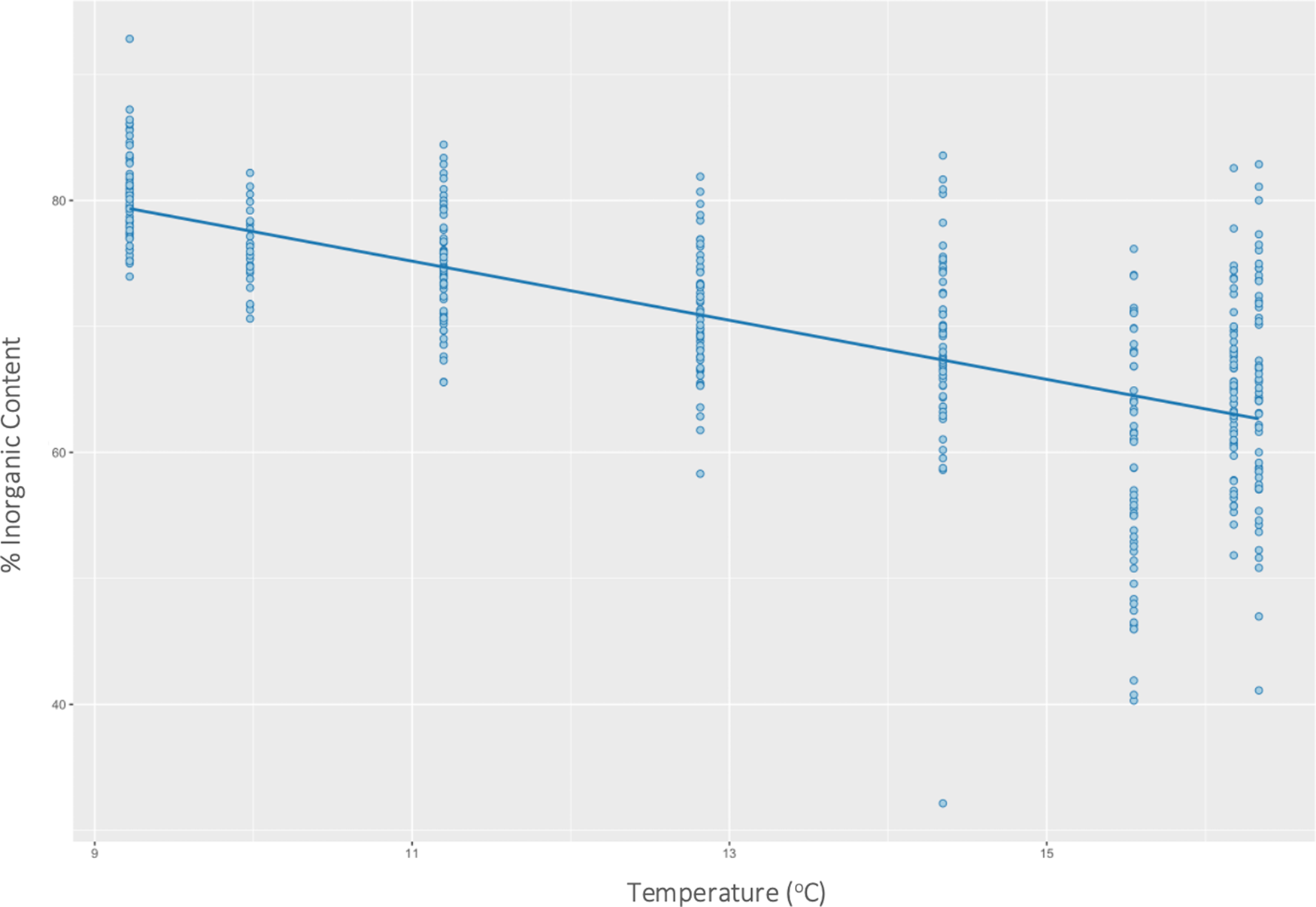
Fig. 2. Effects of temperature on the inorganic content of Halichondria panicea at both sites combined.
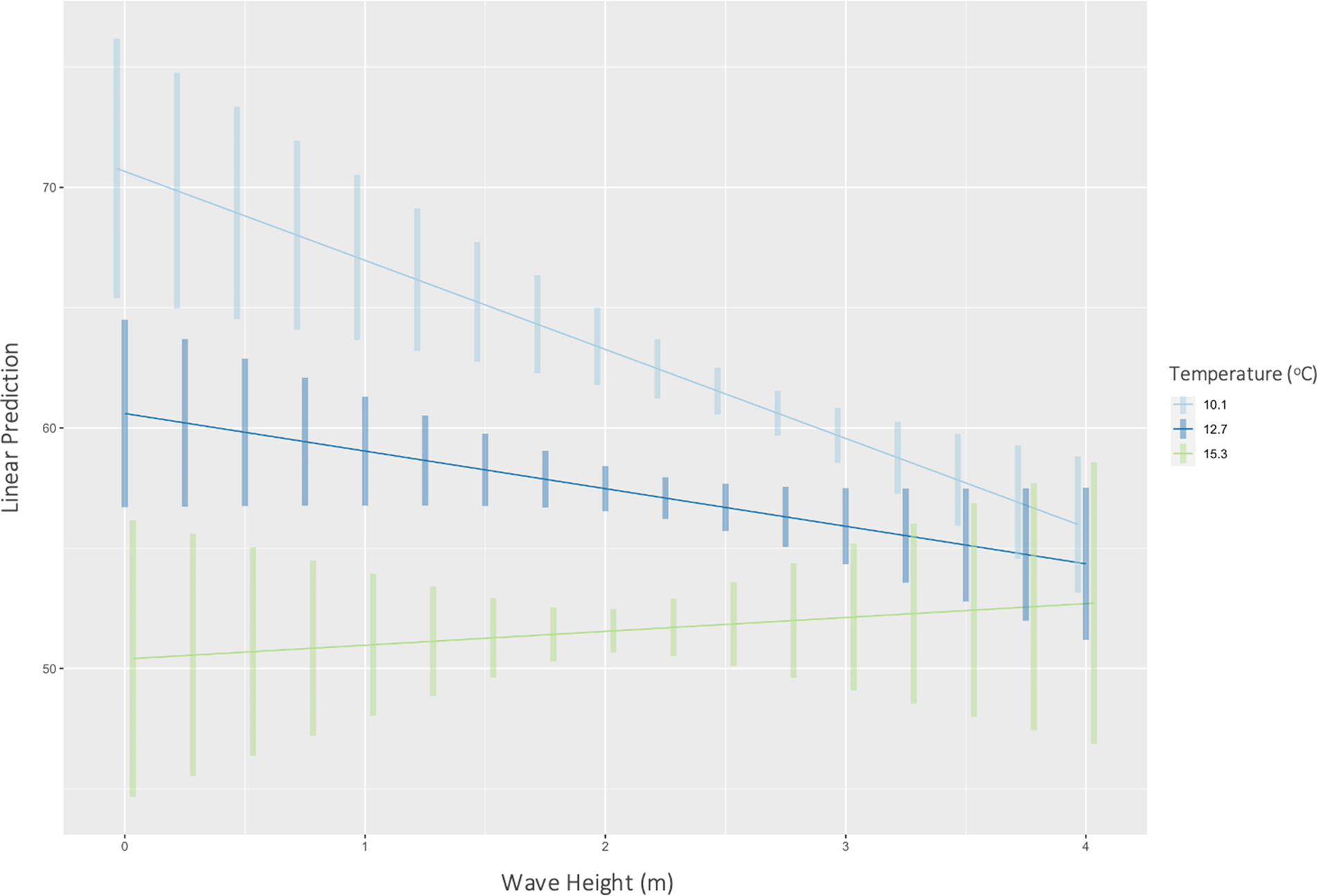
Fig. 3. Predicted correlations (with CIs) between wave height and inorganic content of H. perlevis at mean (12.7°C), mean – 1 SD (10.1°C), and mean + 1 SD (15.3°C) temperatures at both sites.
Discussion
The ability of organisms to make changes to their morphology over short time scales allows them to react to seasonal changes in environmental conditions and increase their chances of survival. Palumbi (Reference Palumbi1984) previously described spatial variation in the relationship between wave action and stiffness/strength in the sponge H. panicea by transplanting sponges from high to low energy environments and vice versa. We aimed to explore whether our two sponge species can exhibit temporal variation in organic and inorganic content within the same environment. We found that both H. perlevis and H. panicea displayed seasonal plasticity in their organic/inorganic content. Both exhibited short-term changes that were correlated with local environmental conditions. This suggests that these two species can acclimate over short time frames to gradual changes in environmental conditions.
As predicted, proportions of inorganic content were generally higher in the winter months. They lowered in the summer months, showing a positive relationship with wave height data and a negative relationship with temperature. Halichondria panicea showed less variation in inorganic content between sites than H. perlevis, indicating that H. panicea is less responsive to, or at least less influenced by localized environmental change with respect to inorganic content (most likely indicative of spicule size and number) or organic tissue content. There is a possibility that inorganic content does not directly correlate with spicule size or number, as many sponges have been found to incorporate foreign material into their tissues to strengthen their skeletons (Cerrano et al., Reference Cerrano, Calcinai, Di Camillo, Valisano, Bavestrello, Custódi, Lôbo-Hajdu, Hajdu and Muricy2007). However, no evidence suggests that this is a strategy used by the two sponge species considered in our study.
We also observed a rapid drop in the organic content of H. perlevis from April to May at Clarach, which did not follow the general month to month trend and could be indicative of a reproductive event (see Figure 1B). At Clarach organic content generally increased from March to July, but declined in May, which could have resulted from a gamete or larval release and is consistent with previously reported reproductive timings of this species (Gaino et al., Reference Gaino, Frine and Giuseppe2010). However, this only occurred at one site, suggesting that local environmental conditions could have been more favourable for reproduction at Clarach than at Borth. Nonetheless, the proximity of these two sites makes it difficult to disentangle what conditions could be influencing the timing of this potential reproductive event. It would be important to collect local environmental data for the specific sampling sites over time for future studies. A reproductive event occurring during lower wave action and higher temperature may indicate that these are optimum conditions for reproduction due to higher food availability and higher larval settlement potential. As this potential reproductive event happened in May for H. perlevis, and we did not begin sampling of H. panicea until July, it is possible we would have seen a similar pattern in H. panicea if this species responds to the same reproductive cues as H. perlevis. To ascertain whether this is the case H. panicea sampling would have to be carried out during the earlier months of the year.
Temperature differences accounted for more variation in the inorganic content of H. panicea than wave height, which contrasts with H. perlevis, which was influenced by both wave height and temperature. It is possible that as H. panicea was only sampled from July to February we are not seeing the real influence of wave height, as it could have a stronger correlation with inorganic content in the months that were not sampled. To explore the influences of wave height and temperature further, H. panicea needs to be surveyed throughout the whole year. As expected, the inorganic content of H. perlevis was influenced by both wave height and temperature, and these two predictors interacted with one another. Wave height and inorganic content became less negatively correlated as temperature increased, to the point where they were weakly positively correlated at high temperatures. Based on previous studies (Palumbi, Reference Palumbi1984; Schönberg & Barthel, Reference Schönberg and Barthel1997, 2000; Mercurio et al., Reference Mercurio, Corriero, Scalera Liaci and Gaino2000) we predicted wave action to have the strongest effects on inorganic content, and this is the highest in winter and spring when temperatures are lowest, in keeping with our results. It is important to note that an increase in the proportion of inorganic content does not necessarily indicate an increase in size and number of spicules, but could indicate a drop in organic content levels (Schönberg & Barthel, Reference Schönberg and Barthel1997).
It is possible that changes in organic and inorganic content could be due to factors other than morphological acclimation or reproductive timing, for example as a response to predation (Knowlton & Highsmith, Reference Knowlton and Highsmith2000, Reference Knowlton and Highsmith2005) or disease (Webster, Reference Webster2007). However, there is no evidence that sponge predation changes seasonally at this site, and we found no specific reports of natural sponge disease on either species (and no disease was evident when samples were collected). Increased temperature and decreased flow rate have been shown to increase bacterial populations on H. panicea in induced laboratory conditions (Hummel et al., Reference Hummel, Sepers, de Wolf and Melissen1988). However if this were the cause of a decrease in organic content this would likely happen in the summer months.
In addition, seasonal changes in food availability may also explain the changes in sponge organic/inorganic tissue content. The patterns reported may be a consequence of other processes rather than morphological acclimation. Since less food is likely to be available during the winter months (Duckworth & Battershill, Reference Duckworth and Battershill2001; Lüskow et al., Reference Lüskow, Riisgård, Solovyeva and Brewer2019), sponges may have to consume internal resources to meet metabolic demand, thus reducing organic content. As the temperature is likely to correlate with food availability, it is again challenging to disentangle these potential drivers. There is also the potential that nutrient fluxes and food availability in the water column could influence spicule growth rate and therefore influence inorganic content (Maldonado et al., Reference Maldonado, Ribes and van Duyl2012), but no data on nutrient fluxes for the region are currently available so we were unable to test this. Regardless, we believe based on our results coupled with previous studies (Palumbi, Reference Palumbi1984, Reference Palumbi1986; Bell & Barnes, Reference Bell and Barnes2000; Schönberg, Reference Schönberg2021) that the increased inorganic content during winter months is a response to the need for protection from the damaging effects of wave action. In addition, it is possible that since temperature has been shown to influence reproductive timing (Witte et al., Reference Witte, Barthel and Tendal1994; Xue et al., Reference Xue, Zhang and Zhang2009), and wave action has been shown to influence the stiffness of sponges (Palumbi, Reference Palumbi1984), we see both effects co-occurring. Further experiments will be needed to enable these effects to be disentangled conclusively.
Acknowledgements
We are grateful for the assistance of Adrian Dowding in the processing of sponge samples. Aberystwyth University provided funding.





