Published online by Cambridge University Press: 10 August 2022
The new sodalite-group mineral bolotinaite, ideally (Na7□)(Al6Si6O24)F⋅4H2O, was discovered in a volcanic ejectum of trachitoid sanidinite collected from the In den Dellen (Zieglowski) pumice quarry, Laach Lake (Laacher See) palaeovolcano, Eifel region, Rhineland-Palatinate, Germany. The associated minerals are sanidine, nepheline, annite and zircon. Bolotinaite occurs as isolated interpenetration prismatic twins on (111) up to 1.3 mm long, complex twins, and rare non-twinned rhombic dodecahedra up to 0.2 mm across. The colour of bolotinaite is pale yellow to pinkish coloured, the streak is white and the lustre is vitreous. Weak orange–yellow fluorescence under longwave ultraviolet radiation (λ = 330 nm) is due to the presence of trace amounts of the S2•– radical anion. Bolotinaite is brittle, with a Mohs’ hardness of 5. No cleavage is observed. The fracture is uneven. D(meas) = 2.27(2) g⋅cm–3, D(calc) = 2.291 g⋅cm–3. Bolotinaite is optically isotropic, with n = 1.488(2) (λ = 589 nm). The chemical composition is (wt.%, electron microprobe, CO2 determined by quantitative IR spectroscopy analysis, H2O calculated from the empirical formula with four H2O molecules per formula unit): Na2O 18.30, K2O 3.87, CaO 0.57, Al2O3 28.85, SiO2 37.97, CO2 1.66, SO3 1.37, F 1.60, Cl 0.57, 2.22, H2O 7.21, –O≡(F,Cl) –0.80, total 101.17. The empirical formula is (Na5.92K0.82Ca0.10H0.08)(Si6.33Al5.67O24)(SO4)0.17F0.84Cl0.16(H2O)3.96(CO2)0.38. A high content of H2O and the presence of CO2 molecules and H+ cations as well as trace amounts of S2•– are confirmed by means of infrared and Raman spectroscopy. The crystal structure was determined using single-crystal X-ray diffraction data and refined to R = 0.0335. Bolotinaite is cubic, space group I$\bar{4}$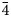 3m, with a = 9.027(1) Å, V = 735.7(2) Å3 and Z = 1. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %) (hkl)] are: 6.36 (47) (110), 4.502 (10) (200), 3.679 (100) (211), 2.851 (28) (310), 2.603 (29) (222) and 2.126 (18) (330). The mineral is named in honour of the Russian crystallographer and crystal chemist Dr. Nadezhda Borisovna Bolotina (b. 1949).
3m, with a = 9.027(1) Å, V = 735.7(2) Å3 and Z = 1. The strongest lines of the powder X-ray diffraction pattern [d, Å (I, %) (hkl)] are: 6.36 (47) (110), 4.502 (10) (200), 3.679 (100) (211), 2.851 (28) (310), 2.603 (29) (222) and 2.126 (18) (330). The mineral is named in honour of the Russian crystallographer and crystal chemist Dr. Nadezhda Borisovna Bolotina (b. 1949).
Associate Editor: Juraj Majzlan