Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Kolitsch, U.
and
Giester, G.
2001.
Revision of the crystal structure of ulrichite, CaCu2+(UO2)(PO4)2·4H2O.
Mineralogical Magazine,
Vol. 65,
Issue. 6,
p.
717.
Krivovichev, S. V.
and
Burns, P. C.
2004.
Synthesis and Crystal Structure of Cs4[UO2(CO3)3].
Radiochemistry,
Vol. 46,
Issue. 1,
p.
12.
Frost, Ray L.
Erickson, Kristy L.
Weier, Matt L.
Carmody, Onuma
and
Čejka, Jiří
2005.
Raman spectroscopic study of the uranyl tricarbonate mineral liebigite.
Journal of Molecular Structure,
Vol. 737,
Issue. 2-3,
p.
173.
Amayri, Samer
Reich, Tobias
Arnold, Thuro
Geipel, Gerhard
and
Bernhard, Gert
2005.
Spectroscopic characterization of alkaline earth uranyl carbonates.
Journal of Solid State Chemistry,
Vol. 178,
Issue. 2,
p.
567.
Burns, Peter C.
2007.
Structural Chemistry of Inorganic Actinide Compounds.
p.
1.
Villars, P.
Cenzual, K.
Daams, J.
Gladyshevskii, R.
Shcherban, O.
Dubenskyy, V.
Melnichenko-Koblyuk, N.
Pavlyuk, O.
Savysyuk, I.
Stoyko, S.
and
Sysa, L.
2008.
Structure Types. Part 6: Space Groups (166) R-3m - (160) R3m.
Vol. 43A6,
Issue. ,
p.
229.
Čejka, Jiří
Sejkora, Jiří
Plášil, Jakub
Bahfenne, Silmarilly
Palmer, Sara J.
and
Frost, Ray L.
2010.
Raman spectroscopic study of the uranyl carbonate mineral čejkaite and its comparison with synthetic trigonal Na4[UO2(CO3)3].
Journal of Raman Spectroscopy,
Vol. 41,
Issue. 4,
p.
459.
Grenthe, Ingmar
Drożdżyński, Janusz
Fujino, Takeo
Buck, Edgar C.
Albrecht-Schmitt, Thomas E.
and
Wolf, Stephen F.
2010.
The Chemistry of the Actinide and Transactinide Elements.
p.
253.
Schindler, M.
Hawthorne, Frank C.
Mandaliev, P.
Burns, Peter C.
and
Maurice, P. A.
2011.
An integrated study of uranyl mineral dissolution processes: etch pit formation, effects of cations in solution, and secondary precipitation.
Radiochimica Acta,
Vol. 99,
Issue. 2,
p.
79.
Vallet, Valérie
Wahlgren, Ulf
and
Grenthe, Ingmar
2012.
Probing the Nature of Chemical Bonding in Uranyl(VI) Complexes with Quantum Chemical Methods.
The Journal of Physical Chemistry A,
Vol. 116,
Issue. 50,
p.
12373.
Olds, Travis A.
Sadergaski, Luke R.
Plášil, Jakub
Kampf, Anthony R.
Burns, Peter C.
Steele, Ian M.
Marty, Joe
Carlson, Shawn M.
and
Mills, Owen P.
2017.
Leószilárdite, the first Na,Mg-containing uranyl carbonate from the Markey Mine, San Juan County, Utah, USA.
Mineralogical Magazine,
Vol. 81,
Issue. 5,
p.
1039.
Gurzhiy, Vladislav V.
Krzhizhanovskaya, Maria G.
Izatulina, Alina R.
Sigmon, Ginger E.
Krivovichev, Sergey V.
and
Burns, Peter C.
2018.
Structure Refinement and Thermal Stability Studies of the Uranyl Carbonate Mineral Andersonite, Na2Ca[(UO2)(CO3)3]·(5+x)H2O.
Minerals,
Vol. 8,
Issue. 12,
p.
586.
Kalashnyk, Nataliya
Perry, Dale L.
Massuyeau, Florian
and
Faulques, Eric
2018.
Exploring Optical and Vibrational Properties of the Uranium Carbonate Andersonite with Spectroscopy and First-Principles Calculations.
The Journal of Physical Chemistry C,
Vol. 122,
Issue. 13,
p.
7410.
Colmenero, Francisco
Plášil, Jakub
and
Sejkora, Jiří
2020.
The crystal structures and mechanical properties of the uranyl carbonate minerals roubaultite, fontanite, sharpite, widenmannite, grimselite and čejkaite.
Inorganic Chemistry Frontiers,
Vol. 7,
Issue. 21,
p.
4197.
Gurzhiy, Vladislav V.
Kalashnikova, Sophia A.
Kuporev, Ivan V.
and
Plášil, Jakub
2021.
Crystal Chemistry and Structural Complexity of the Uranyl Carbonate Minerals and Synthetic Compounds.
Crystals,
Vol. 11,
Issue. 6,
p.
704.
Pyrch, Mikaela Mary F.
Bjorklund, Jennifer L.
Williams, James M.
Kasperski, Maguire
Mason, Sara E.
and
Forbes, Tori Z.
2022.
Investigations of the Cobalt Hexamine Uranyl Carbonate System: Understanding the Influence of Charge and Hydrogen Bonding on the Modification of Vibrational Modes in Uranyl Compounds.
Inorganic Chemistry,
Vol. 61,
Issue. 38,
p.
15023.
Rodríguez-Villagra, N.
Bonales, L. J.
Milena-Pérez, A.
and
Galán, H.
2023.
A snapshot review on uranyl secondary phases formation in aqueous systems.
MRS Advances,
Vol. 8,
Issue. 6,
p.
207.
Fairley, Melissa
Sigmon, Ginger E.
and
LaVerne, Jay A.
2023.
Solid-State Transformation of Uranyl Peroxide Materials through High-Level Irradiation.
Inorganic Chemistry,
Vol. 62,
Issue. 48,
p.
19780.
Gurzhiy, V. V.
Nazarchuk, E. V.
Tagirova, Y. G.
and
Krivovichev, S. V.
2025.
Results of 25-Year Studies of Uranium Compounds at the Department of Crystallography of St. Petersburg State University.
Crystallography Reports,
Vol. 70,
Issue. 2,
p.
235.
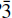 c1 and Z = 4. The structure was refined on the basis of F 2 (wR2 = 4.2%) for all unique data collected using Mo-Kα X-radiation and a CCD-based detector. The final R1 was 2.0%, calculated for 534 unique observed (F o ≥ 4σF ) reflections, and the goodness-of-fit (S) was 0.91. The structure contains a uranyl tricarbonate cluster composed of a uranyl hexagonal bipyramid that shares three equatorial edges with CO3 triangles. The uranyl tricarbonate clusters are connected through NaO6 and NaO5 polyhedra, forming a heteropolyhedral framework structure. This compound may be related to a uranyl carbonate phase with the same composition which has been reported as an alteration phase on the surface of Chernobyl ‘lava’, and as a mineral in the Jachymov ore district, Czech Republic.
c1 and Z = 4. The structure was refined on the basis of F 2 (wR2 = 4.2%) for all unique data collected using Mo-Kα X-radiation and a CCD-based detector. The final R1 was 2.0%, calculated for 534 unique observed (F o ≥ 4σF ) reflections, and the goodness-of-fit (S) was 0.91. The structure contains a uranyl tricarbonate cluster composed of a uranyl hexagonal bipyramid that shares three equatorial edges with CO3 triangles. The uranyl tricarbonate clusters are connected through NaO6 and NaO5 polyhedra, forming a heteropolyhedral framework structure. This compound may be related to a uranyl carbonate phase with the same composition which has been reported as an alteration phase on the surface of Chernobyl ‘lava’, and as a mineral in the Jachymov ore district, Czech Republic.
