Published online by Cambridge University Press: 05 July 2021
The new mineral, zvěstovite-(Zn), ideally Ag6(Ag4Zn2)As4S13, was found in quartz–baryte gangue at the mine dump of the abandoned small deposit of Zvěstov, central Bohemia, Czech Republic. Zvěstovite-(Zn) is associated with tennantite-(Zn), tetrahedrite-(Zn), argentotennantite-(Zn), acanthite and supergene azurite and malachite. The new mineral occurs as rare relic anhedral grains rimmed by acanthite, up to 100 μm in size. Zvěstovite-(Zn) is grey, Mohs hardness is ca. 3½–4, in agreement with other members of the tetrahedrite group; the calculated density is 5.16 g.cm–3. In reflected light, zvěstovite-(Zn) is grey with a greenish tint, without bireflectance, pleochroism or anisotropy. Deep red internal reflections are ubiquitous. Reflectance values of zvěstovite-(Zn) in air (R%) are: 28.5 at 470 nm, 26.9 at 546 nm, 25.5 at 589 nm and 23.8 at 650 nm. The empirical formula for zvěstovite-(Zn), based on electron-microprobe analyses (n = 4), is Ag6.27[(Ag3.90Cu0.38)Σ4.28(Zn1.60Fe0.09Cd0.03)Σ1.72]Σ6.00(As2.26Sb1.48)Σ3.74S12.50. The ideal formula is Ag6(Ag4Zn2)As4S13, which requires (in wt.%) Ag 56.01, Zn 6.79, As 15.56 and S 21.64, total 100.00. Zvěstovite-(Zn) is cubic, I$\bar{4}$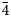 3m, with unit-cell parameters: a = 10.850(2) Å, V = 1277.3(8) Å3 and Z = 2. The strongest reflections of the calculated powder X-ray diffraction pattern [d, Å (I) (hkl)] are: 3.1321(100) (222), 2.7125(21) (400), 1.9809(11) (521), 1.9180(31) (440) and 1.6357(15) (622). According to the single-crystal X-ray diffraction data (Robs = 0.051), the crystal structure of zvěstovite-(Zn) agrees with the general features of the members of the tetrahedrite group. Zvěstovite-(Zn) is named after its type locality, Zvěstov; the suffix indicates the dominant divalent C-constituent, according to the approved nomenclature of the tetrahedrite group. It is the As-isotype of rozhdestvenskayaite-(Zn). The mineral and its name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2020-061).
3m, with unit-cell parameters: a = 10.850(2) Å, V = 1277.3(8) Å3 and Z = 2. The strongest reflections of the calculated powder X-ray diffraction pattern [d, Å (I) (hkl)] are: 3.1321(100) (222), 2.7125(21) (400), 1.9809(11) (521), 1.9180(31) (440) and 1.6357(15) (622). According to the single-crystal X-ray diffraction data (Robs = 0.051), the crystal structure of zvěstovite-(Zn) agrees with the general features of the members of the tetrahedrite group. Zvěstovite-(Zn) is named after its type locality, Zvěstov; the suffix indicates the dominant divalent C-constituent, according to the approved nomenclature of the tetrahedrite group. It is the As-isotype of rozhdestvenskayaite-(Zn). The mineral and its name have been approved by the Commission on New Minerals, Nomenclature and Classification of the International Mineralogical Association (IMA2020-061).
Associate Editor: František Laufek