Introduction
Eudialyte-group minerals (EGM) are Na-Ca-Zr-cyclosilicates that accommodate many elements in their complex trigonal crystal structure. In addition to the essential constituents Na, Ca, Zr and Si, they host significant amounts of Fe, Mn, REE, Y, Nb, Hf, Ti, K, Sr and Ti as well as Cl, F, H2O and OH groups (Johnsen and Grice, Reference Johnsen and Grice1999). Consequently, the eudialyte group encompasses a wide range of minerals of varying compositions and space groups (Rastsvetaeva and Chukanov, Reference Rastsvetaeva2012), which at present includes over 28 species accepted by the International Mineralogical Association (IMA), reported from c. 105 localities worldwide (Marks and Markl, Reference Marks and Markl2017).
Eudialyte-group minerals, their alteration products and associated mineral assemblages are of significant economic interest as they provide potential low-cost resources for elements such as Zr, REE, Nb and Ta. These metals have a wide variety of uses in modern technologies, ranging from durable alloys, permanent magnets, catalysts and energy-efficient lighting phosphors to rechargeable batteries, and thus crucial enablers of the clean energy and transport transition. Rare earth elements, in particular, are considered critical metals due to challenges in their supply chain and a projected growth in demand (Chakhmouradian and Wall, Reference Chakhmouradian and Wall2012; Hatch, Reference Hatch2012; European Commission, 2017; Goodenough et al., Reference Goodenough, Wall and Merriman2017; Roskill, Reference Roskill2018; Adamas Intelligence, Reference Castilloux2019). Significant deposits of EGM are found in peralkaline igneous complexes such as: Ilímaussaq (Greenland); Norra Kärr (Sweden); Lovozero and Khibina (Russia); Kipawa (Canada); and Pajarito (USA) (e.g. Mariano and Mariano Jr, Reference Mariano and Mariano2012; Sjöqvist et al., Reference Sjöqvist, Cornell, Andersen, Erambert, Ek and Leijd2013; Machacek and Kalvig, Reference Machacek and Kalvig2016; Goodenough et al., Reference Goodenough, Schilling, Jonsson, Kalvig, Charles, Tuduri, Deady, Sadeghi, Schiellerup and Müller2016; Smith et al., Reference Smith, Moore, Kavecsánszki, Finch, Kynicky and Wall2016; Marks and Markl, Reference Marks and Markl2017; Borst et al., Reference Borst, Friis, Nielsen and Waight2018). Eudialyte represents a relatively low-grade ore mineral (~1–10 wt.% total REE 2O3, ~1 wt.% of Nb2O5 and <0.5 wt.% Ta2O5) compared to carbonatite-hosted REE ore minerals, but their economic importance is enhanced by relatively high proportions of the more valuable (i.e. more critical) heavy rare earth elements (HREE) relative to the light rare earth elements (LREE) (Fryer and Edgar, Reference Fryer and Edgar1977; Binnemans et al., Reference Binnemans, Jones, Müller and Yurramendi2018), as well as relatively low U and Th contents (Schilling et al., Reference Schilling, Wu, McCammon, Wenzel, Marks, Pfaff, Jacob and Markl2011) and ease of extraction through magnetic separation (Goodenough et al., Reference Goodenough, Wall and Merriman2017; Paulick and Machacek, Reference Paulick and Machacek2017). Recent efforts to improve the metallurgical processing of eudialyte focused on resolving issues with the formation of silica gels which hinders metal extraction. Innovative multi-step leaching techniques have been developed and promise potential for upscaling, though economic viability has yet to be demonstrated at industry scales (Stark et al., Reference Stark, Silin and Wotruba2016; Balomenos et al., Reference Balomenos, Davris, Deady, Yang, Panias, Friedrich, Binnemans, Seisenbaeva, Dittrich, Kalvig and Paspaliaris2017; Davris et al., Reference Davris, Stopic, Balomenos, Panias, Paspaliaris and Friedrich2017; Voßenkaul et al., Reference Voßenkaul, Birich, Müller, Stoltz and Friedrich2017).
At the heart of eudialyte's commercial value is its relatively flat chondrite-normalised REE profile (Chakhmouradian and Wall, Reference Chakhmouradian and Wall2012, Fryer and Edgar, Reference Fryer and Edgar1977). Partitioning of REE in minerals typically produces parabolic or curved profiles (due to partitioning behaviour as a function of ionic radius, e.g. Blundy and Wood, Reference Blundy and Wood2003) and the unusually flat to HREE-enriched profiles in EGM hints at substitutions into multiple sites, site ordering and/or defect clustering. Furthermore, the open literature is inconsistent as to whether REE substitute on the Na, Ca or Zr sites, or a combination of these (Johnsen and Grice, Reference Johnsen and Grice1999; Johnsen et al., Reference Johnsen, Grice and Gault2001; Rastsvetaeva, Reference Rastsvetaeva2007; Pfaff et al., Reference Pfaff, Krumrei, Marks, Wenzel, Rudolf and Markl2008; Möller and Williams-Jones, Reference Möller and Williams-Jones2016). Such considerations are more than academic – EGM deposits are commonly altered (Mitchell and Liferovich, Reference Mitchell and Liferovich2006; Borst et al., Reference Borst, Friis, Andersen, Nielsen, Waight and Smit2016; Möller and Williams-Jones, Reference Möller and Williams-Jones2017; Estrade et al., Reference Estrade, Salvi and Béziat2018; van de Ven et al., Reference van de Ven, Borst, Davies, Hunt and Finch2019) and some replacement processes are topotactic (i.e. retaining structural templates from the primary structure to the secondary). Furthermore, the silica-gels that form during eudialyte dissolution (e.g. Voßenkaul et al., Reference Voßenkaul, Birich, Müller, Stoltz and Friedrich2017; Davris et al., Reference Davris, Stopic, Balomenos, Panias, Paspaliaris and Friedrich2017) may preserve structural states from the original eudialyte. There is therefore a commercial imperative that REE substitution in eudialyte is fully understood.
Here we use X-ray absorption spectroscopy (XAS), i.e. XANES (X-ray absorption near-edge structure) and EXAFS (extended X-ray absorption fine structure) to probe the local coordination environment of REE in eudialyte-group minerals. We measure Y K-edge and Nd L 3-edge absorption spectra as proxies for the heavy and light REE, respectively. The data are used to quantify REE coordination numbers and nearest-neighbour bond distances to provide a more detailed understanding of REE substitution mechanisms in the EGM crystal structure. We analyse the results using lattice strain partitioning models and extrapolate the partitioning behaviour for the lanthanides we have not measured directly. In doing so, we provide insights into the flat-to-inclined heavy REE profiles that make eudialyte such an attractive resource.
Crystal structure of the eudialyte group
Minerals of the trigonal eudialyte aristotype are composed of a heteropolyhedral framework of interlayered three- and nine-membered rings of Si tetrahedra [Si3O9 and Si9O27] and six-membered rings of edge-shared Ca octahedra [Ca6O24], joined together by isolated Zr octahedra. The layers follow a TZTM ordering along the c axis, where T denotes the three- and nine-membered [Si3O9] and [Si9O27] rings, Z denotes layers of discrete Zr and/or Ti octahedra and M represents layers of six-membered rings of Ca octahedra (Rastsvetaeva, Reference Rastsvetaeva2007). Spaces within the framework are filled with polyhedra of alkali, alkaline-earth and transitional-metal cations (valences + 1 to + 6) and volatile groups (Cl–, F–, OH–, SO4–, H2O and H3O+). Iron and manganese predominantly occupy 4- or 5-coordinated M2 sites, respectively, in planar squares or five vertex-pyramids between the six-membered rings (Guiseppetti et al., Reference Guiseppetti, Mazzi and Tadini1971; Johnsen and Grice, Reference Johnsen and Grice1999; Rastsvetaeva and Chukanov, Reference Rastsvetaeva and Chukanov2012; Rastsvetaeva, Reference Rastsvetaeva2007). Ordering of elements on the various sites gives rise to a wide range of EGM compositions and structural hettotypes, predominantly within the R3m, R  $\bar{3}$m and R3 space groups (Rastsvetaeva, Reference Rastsvetaeva2007, Rastsvetaeva and Chukanov, Reference Rastsvetaeva and Chukanov2012).
$\bar{3}$m and R3 space groups (Rastsvetaeva, Reference Rastsvetaeva2007, Rastsvetaeva and Chukanov, Reference Rastsvetaeva and Chukanov2012).
The IMA-accepted formula for the eudialyte group is: N 15–16[M1]6[M2]3[M3][M4]Z 3Si24O66–73(Θ)0–9(X)2, where: N = Na+, K+, Sr2+, Ca2+, REE 3+, □ (vacancy), Ba2+, Mn2+ or H3O+; M1 = Ca2+, REE 3+, Mn2+, Fe2+, Na+, Sr2+; M2 = Fe2+, Mn2+, Na+, □, H3O+, Zr4+, Ta5+, Ti4+, K+, Ba2+ or Fe3+; M3/4 = Si4+, Al3+, Nb5+, Ti4+, W6+ or Na+, Z = Zr4+, Hf4+, Ti4+ or Nb5+; Θ = H2O, OH–, O2–, CO32–, SO42– or SiO44–; and X = Cl–, F– or OH– (Johnsen et al., Reference Johnsen, Ferraris, Gault, Grice, Kampf and Pekov2003).
Empirical formulae are calculated on the basis of 29 cations for the sum of Si, Al, Zr, Ti, Hf, Nb, W and Ta. At present, 200 years after eudialyte was first reported (Stromeyer, Reference Stromeyer1819), the eudialyte group comprises 28 independent IMA-approved mineral species, and new ones are described regularly (e.g. Rastsvetaeva et al., Reference Rastsvetaeva, Aksenov and Rozenberg2015, Reference Rastsvetaeva, Rozenberg, Chukanov and Aksenov2017). It is worth noting that the number of crystallographically non-equivalent sites increases in non-centrosymmetric space groups. Common species of EGM include eudialyte sensu stricto, kentbrooksite, ferrokentbrooksite, alluaivite, oneillite and (Mn-) raslakite (Table 1), with natural samples showing solid solution between end-members.
Table 1. IMA approved members of the Eudialyte Group mentioned in the text.

aThe REE are on the N4 subsite; bmembers of the R3 space groups (oneillite sub-group) have two ordered M1 subsites, M1a and M1b, respectively, in the M16O36 rings; cM2 site coordination is indicated by the superscript.
Of the many trace elements that substitute into eudialyte-group minerals, the REE are particularly significant, and can reach up to 10 wt.% REE 2O3 (Grice and Gault, Reference Grice and Gault2006). However, their exact location has been unclear. In eudialyte s.s. the REE are inferred to substitute for Ca on the M1 site, while in kentbrooksite, oneillite and raslakite, the REE are inferred to occupy both the N and M1 sites. Johnsenite-(Ce) and zirsilite-(Ce) are the only ‘true’ REE-species, where REE (dominantly light) dominate the N4 site (Table 1). No direct analysis of lanthanide substitution has been performed on the more common EGM members. Furthermore, it is unclear whether the light and heavy REE partition into different sites as a function of their varying ionic radii.
Approach and hypothesis
Using XAS we perform the first direct measurements to probe the coordination sphere of Y and Nd atoms in the structure of common EGM, particularly to test whether heavy and light REE occupy different structural sites. If both Y3+ and Nd3+ substitute for Ca2+ on the M1 site, the data will yield best fits in 6-fold coordination with interatomic bond distances approaching 2.35 Å. Significantly longer bond distances (c. 2.6 Å) and larger mean-square relative displacement Debye-Waller factors (σ2, attenuation of X-ray scattering, indicative of greater structural disorder) would imply that REE are occupying the larger, low symmetry, multi-coordinated N sites. Smaller bond distances, Debye–Waller factors and a second coordination sphere fitting to 6 Si and 10 Na between 3.6–3.8 Å may suggest that Y (0.9 Å) and, by inference, similarly sized heavy REE, substitute for Zr on the octahedral Z site. The presence of REE in multiple sites would be inferred from bond distances inconsistent with any of the sites and large Debye–Waller factors inconsistent with a single site. Additional information on the nature and distance of elements in the second coordination sphere are extracted from the EXAFS signal to further constrain the local structure of REE in EGM.
Methods and materials
Sample preparation
X-ray absorption spectra were collected for EGM from five localities; Ilímaussaq, the NarsaarsukFootnote 1 pegmatite (Greenland); Norra Kärr (Sweden); Kipawa (Canada); and Lovozero (Russia). Sample details and compositional data are provided in Supplementary 1, Table S1 and Table S2 deposited with the Principal Editors of Mineralogical Magazine (see below). The samples are dominantly eudialyte s.s. with minor kentbrooksite components (Ilímaussaq, Narsaarsuk, Norra Kärr), a (Mn,Ca)-ordered variety (Lovozero) and a low Fe, high Ca-Y variety (Kipawa). Most EGM display LREE-enriched rare-earth profiles with flat HREE patterns (Supplementary 1). The most HREE-(particularly Y) enriched sample derives from Kipawa, consistent with published EGM compositions from that complex (Johnsen and Grice, Reference Johnsen and Grice1999). For simplicity we refer to all studied EGM as ‘eudialyte’. In addition, we measured a selection of natural and synthetic REE-standards in which the REE occupy various coordination states and symmetry environments (Tables 2, S1) because few XANES data for REE-(bearing) minerals are available in the literature. Microcrystalline Y-doped monazite-(Nd) was synthesised at the University of St Andrews following procedures described by Friis (Reference Friis2009) (Supplementary 1). Synthetic crystals of Nd-doped YPO4 were provided by Lynn Boatner, ORNL (Boatner, Reference Boatner, Kohn, Rakovan and Hughes2002). Excluding the gem-quality zircon and a diamond blank, samples and standards were ground under ethanol in an agate mortar and checked for phase purity by powder X-ray diffraction (XRD) (University of St Andrews) prior to XAS measurements. Samples were measured as powders or crystals mounted on KAPTON© tape. Single-cation Y and Nd solutions (prepared from Y2O3 in 2% nitric acid, Inorganic Ventures CGY1) were measured after injection into a metallic liquid-sample cell sealed between KAPTON© tape.
XAS data collection
X-ray absorption spectroscopy measurements were carried out at the I18 micro-focus beamline at the Diamond Light Source, a 3 GeV 3rd generation synchrotron facility (Didcot, United Kingdom), and at the SUL-X beamline of the KIT Karlsruhe Light Source, a 2.5 GeV storage ring with typical electron currents of 100 to 150 meV (Karlsruhe Institute for Technology, Germany). The I18 beamline is designed for high spatial resolution analyses of heterogeneous samples within the 2.05–20.5 keV energy range, and set up for μ-X-ray fluorescence mapping, μ-XRD, μ-XANES and μ-EXAFS (see Mosselmans et al., Reference Mosselmans J.F.W., Dent, Cavill, Moreno, Peach, Leicester, Keylock, Gregory, Atkinson and Rosell2009). The SUL-X beamline is set up for the same micro-methods as I18 and uses a 27 pole Wiggler radiation source to cover an energy range of 2.4 to 20 keV. Details of the set-up and measurements at the SUL-X beamline are provided in Supplementary 1.
All measurements were run at room temperature. The EGM samples and standards with medium to low Y and Nd contents were measured in fluorescence mode, whereas REE-rich standards were measured in transmission mode. A 3 µm × 3 µm focussed beam was employed at I18. At SUL-X measurements were run using a moderately focused beam ranging in size from 150 µm by 250 µm to 50 µm × 50 µm (depending on count rates). The energy range was set to include both the X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) regions for the Y K-edge (17,038 eV) and the Nd L 3-edge (6208 eV). Yttrium was chosen because it can be analysed on the K-edge, which has no significant interference from absorption edges of other elements over the measured energy range, and Nd because natural materials contain no Pm, allowing a longer k range in the EXAFS spectrum than other lanthanides.
At I18, Mn (K-edge at 6539 eV) and Zr (K-edge at 17,998 eV) metal foils were measured for the energy calibration of the Nd L 3-edge spectra (6208 eV) and Y K-edge spectra (17,038 eV), respectively. At SUL-X, energy calibration was done by running Y and Mn metal foils between measurements. Detector count rates were checked to ensure measurement within the linear range. The width of the energy interval defining elemental peak areas was optimised to exclude influence from fluorescence signals of neighbouring elements. The Nd L 3-edge XAS region also contains the Ce L 2-edge at 6164 eV (in the pre-edge region) and the Pr L 2-edge at 6440 eV in the EXAFS. Despite tight windowing of the secondary Nd Lα X-rays (minimising the response from CeL 2 and PrL 2) and insertion of Al-foils in the path of the X-ray beam to filter out lower energy X-rays, L 2-edge absorption peaks for Ce and Pr remain visible in most samples. Due to the interference of both CeL 2 and PrL 2 over the relevant k range, the Nd scans were considered unsuitable for quantitative EXAFS refinement. Samples analysed on both beamlines showed no significant differences in spectra.
XAS data processing
Data were processed using the Athena and Artemis Demeter Perl packages for XAS analyses following the IFEFFIT program (Ravel and Newville, Reference Ravel and Newville2005). XAS spectra were normalised to the X-ray source intensity (I o) and the absorption edge step height to yield μ(E), and the absorption edge set to the maximum of the first derivative. Backgrounds were removed using the AUTOBK background subtraction algorithm (Newville, Reference Newville2001), using spline k ranges of 0 to 11 and a R bkg value of 1.0–1.1, but carefully avoiding the Ce L 2-edge absorption peak in the pre-edge background of the Nd absorption spectra. Duplicate runs (typically n = 4) were merged to improve signal-to-noise ratios. EXAFS oscillations and phase-shifted Fourier transforms providing radial distribution functions were processed and fitted in Artemis (Ravel and Newville, Reference Ravel and Newville2005). Theoretical scattering paths were calculated using the integrated ATOMS and FEFF6 software using diffraction-based crystal structures available from the American Mineralogist Crystal Structure Database (Downs and Hall-Wallace, Reference Downs and Hall-Wallace2003).
The fitted k range and R range for eudialyte was 2.5–10 Å–1 and 1–4 Å, respectively, to yield comparable fitting parameters and the same number of independent variables supported by the data (N = 2∆k∆R/π). For each fit, the number of fitted parameters (P) are kept to a minimum to ensure a determinacy (N/P) above 2. Refined parameters include the energy offset (ΔE 0 in eV), the mean interatomic distance between the absorber and the scatterer atoms (R Y–O, R Y–Si, R Y–Fe, R Y–Ca, etc in Å) and the mean-square relative displacement Debye-Waller factor per scatterer (σ2 in Å–2). The amplitude reduction factor (S02) was determined from a three-shell EXAFS fit for the Y2O3 reference powder by fixing the coordination numbers for each path (1.00 ± 0.18, R factor 0.024). Coordination numbers (CN) were only refined in initial first shell fits, and subsequently fixed to optimal values to reduce the number of variables and maintain a determinacy of >2. Similarly, ΔE 0 was determined from the first-shell fit and subsequently fixed in the second-shell refinements. All XANES and EXAFS data are given in Tables S3 and S4.
Structural model for REE in EGM
For the eudialyte Y EXAFS refinements we consider three possible sites as credible locations for the REE: (1) the octahedral M1 site; (2) the low-symmetry 9-fold N4 site; or (3) the octahedrally Zr-dominant Z site. The Fe or Mn dominated M2 site is not considered a viable location for the REE because of its 4-fold (planar square) or 5-fold coordination; we know of no synthetic or natural solid in which REE adopt such a coordination state. Rather than attempting ab initio refinement of the Y EXAFS in eudialyte, we modelled the data in turn from the starting point of each of the three possible coordination spheres (M1, N4 and Z) and then allowed the parameters to refine. XRD suggests that the M1 site is a slightly distorted and flattened octahedron with 2 O at ~2.31 and 4 O at ~2.37 Å (Table 3, illustrated in Fig. 1b, Johnsen and Grice, Reference Johnsen and Grice1999). The second candidate for REE and Y substitution is the low symmetry N site, of which there are up to five sub-sites (Johnsen and Grice, Reference Johnsen and Grice1999; Rastsvetaeva, Reference Rastsvetaeva2007) with varying coordinations (6 to 10), and much larger Na–O (and Na–Cl) bond distances of ~2.5–2.8 Å (Fig 1d). Of all N sites, XRD suggests the N4 site is the most likely host for REE, Sr, K or Mn (Johnsen and Grice, Reference Johnsen and Grice1999). The third option, the Z site, is a near regular octahedron comprising 6 oxygens at a distance of 2.08 Å from the central Zr atom (Fig 1c), each shared with a Si atom from a corner-linked [SiO4]-tetrahedron. XRD-inferred bond distances per site (for eudialyte s.s.) are listed in Table 3. Theoretical scattering paths for the various sites were calculated using FEFF6 from structural models for eudialyte from the Ilímaussaq complex and the Narsaarsuk pegmatite from Johnsen and Grice (Reference Johnsen and Grice1999). Crystallographic data (.cif) files were rewritten to full site occupation to allow FEFF6 path calculations. Only single scattering paths are considered.
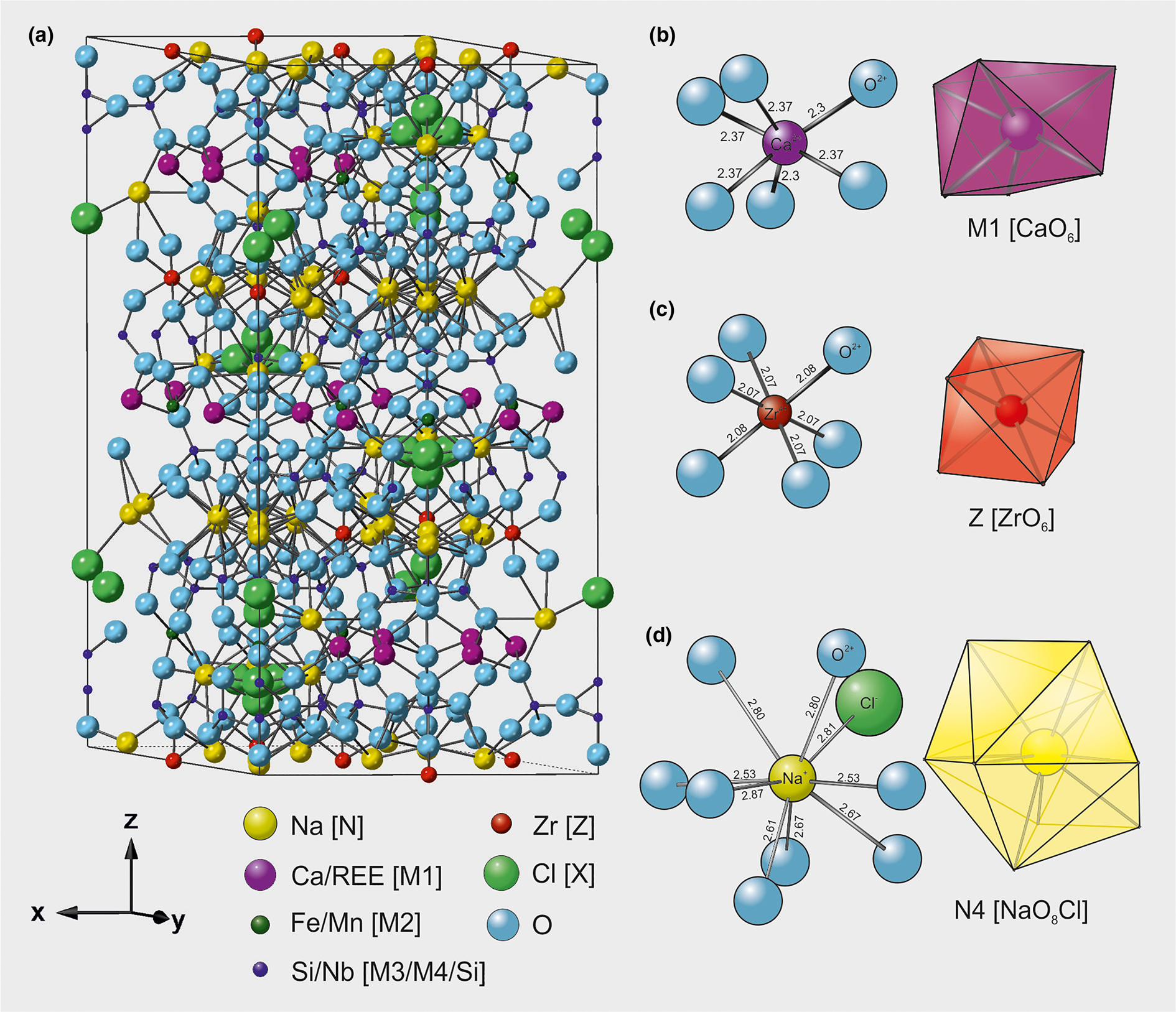
Fig. 1. (a) Unit-cell crystal structure of Ilímaussaq eudialyte s.s. (from Johnsen and Grice, Reference Johnsen and Grice1999) and site geometries for: (b) the octahedral Ca-occupied M1 site; (c) the high symmetry octahedral Z site; and (d) the low symmetry N4 site. Nearest and next-nearest neighbour bond distances per site are listed in Table 3.
Table 2. Eudialyte samples and standards measured in this study.

Table 3. Nearest neighbour bond distances for sites in eudialyte s.s. (Johnsen and Grice, Reference Johnsen and Grice1999; sample #15, Ilimaussaq), used as input parameters for eudialyte Y EXAFS refinements. Refined parameters are shown in Table 5.

Table 4. EXAFS refinement results for selected standards.

aFinal refinements over k-range 2.5 to 9 Å−1, R range variable (see text); b references for the structures used in FEFF6 calculations.
Results
Y K-edge XANES
Normalised Y K-edge XANES for the eudialyte samples and standards are shown in Fig. 2a. All standards display sharp white lines at 17,050–17,052 eV (edge energy determined as the maximum of 1st derivative, indicated with a black line and arrow in Fig. 2), with a narrow peak at ~17,056 eV (feature B, Fig. 2a). The standard spectra show variations in the position and relative height of absorption features labelled A to D (Fig. 2), reflecting different structural states for Y. We observe a potential systematic shift in the position and shape around feature D as a function of coordination number (CN), with the middle of the peak(s) moving to lower energy with increasing CN. From 6- to 9-fold coordinations, the magnitude of this shift is ~10 eV, i.e. the peak is centred at 17,110 eV for phases with Y in 6-coordinated sites, at 17,107 eV for phases with mixed Y coordinations of 6–7–8, at 17,104 eV for 8-fold Y and at 17,100 eV for phases with Y in 9-fold coordination (Fig 2b). Allanite-(Ce), with Y in low-symmetry 9–11-fold sites, is an outlier to this trend. Furthermore, Y XANES for standards in which Y occupies higher point symmetry sites (i.e. zircon, YPO4–Nd, NdPO4–Y and Y2O3) exhibit more pronounced absorption features, in particular a double- or triple-peak around feature D (grey arrows in Fig. 2b), or a shoulder after feature C (Fig. 2a).

Fig. 2. Yttrium K-edge XANES spectra for (a) selected standards and (c) eudialyte. Black line indicates main Y K-edge energy (17,050 eV). 109211 was measured in thin section, other samples as powders. Grey lines labelled A (17,053 eV), B (17,056 eV), C (17,061 eV) and D (17,110 eV) mark characteristic features in the eudialyte spectra. Details of feature D (b and d) reveal shifts in peak shape and position with increasing REE coordination numbers (CN) for the standards (b), and stronger XANES features for minerals where HREE are inferred to occupy higher symmetry sites (Y2O3, zircon, and REE phosphates). The peak position of feature D (grey line) for eudialyte is most comparable to standards where Y is inferred to occupy octahedral sites (CN = 6).
The Y K-edge absorption energy for eudialyte is 17,050 eV (maximum of 1st derivative). The spectra reveal unique XANES features compared to the measured standards. With one exception (AF/99/193, Fig 2b), all eudialyte display a characteristic double-peaked XANES profile, with a small first bump at 17,053 (feature A), which is not identified in the standards, a broad maximum at 17,061 eV (feature C) and a minor downward kink at 17,056 eV (feature B, i.e. where most of the standard XANES have their maxima). The eudialyte spectra furthermore show a broad peak at 17,110 eV (feature D, Fig 2d), representing the first EXAFS oscillation in k space. The position, height and width of feature D in the eudialyte spectra are most consistent with the XANES of standards in which Y is inferred to be in 6-fold coordination (Fig. 2b,d). Further information about the structural state of Y in eudialyte is derived from quantitative refinements of the EXAFS parts of the spectra described in the discussion.
Nd L3-edge XANES
The standards exhibit Nd L 3-XANES spectra with sharp white lines at 6213 eV, narrow peaks, and minor variations in the relative heights and positions of identified XANES features (Fig. 3a). Cerium L 2-edge absorption features appear in the pre-edge region of all natural REE samples. The latter display notable variations in the relative heights of the Ce3+, Ce4+ and Nd3+ absorption peaks, reflecting natural variations in Ce3+/Ce4+ and Ce/Nd ratios (Fig 3). Higher symmetry standards (‘yttrofluorite’, xenotime (YPO4–Nd), Nd-aqueous) show more pronounced Nd XANES features and narrower absorption peaks than the XANES of minerals in which REE are inferred to occupy lower symmetry sites.
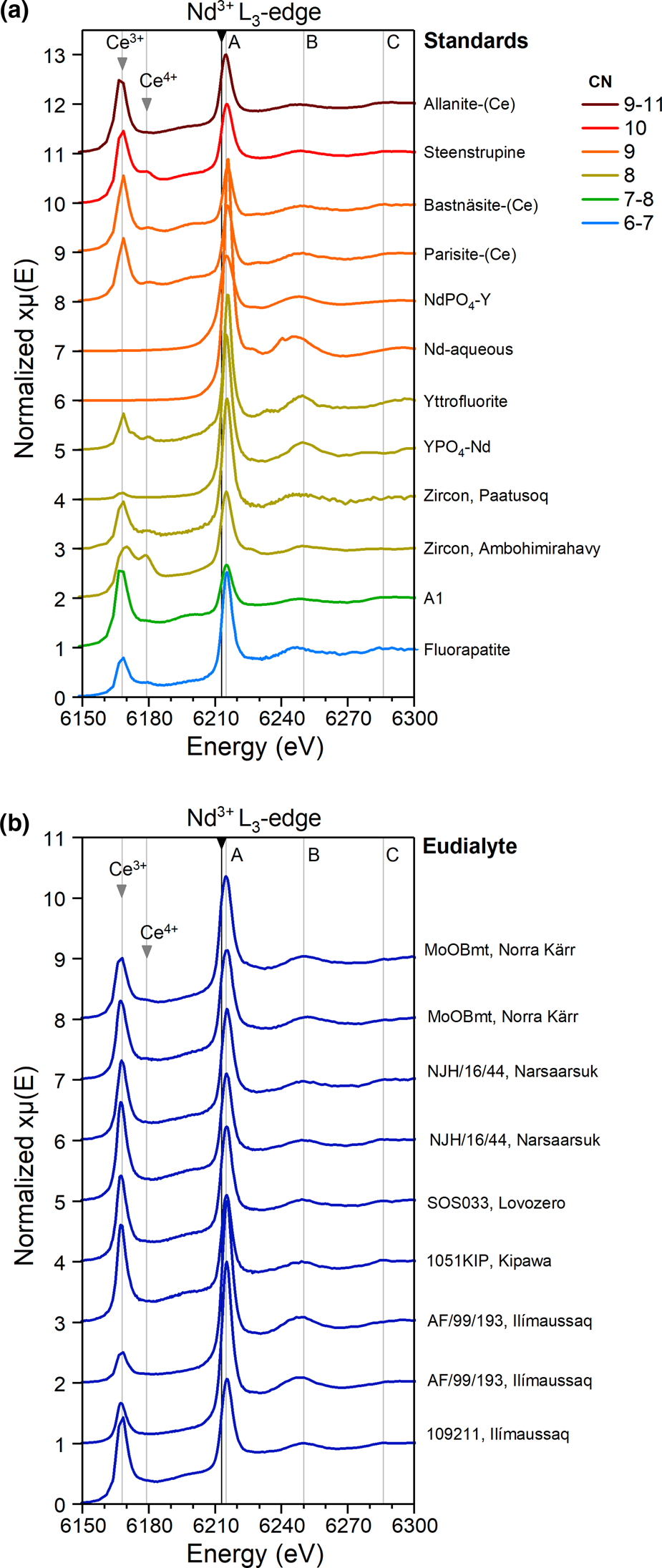
Fig. 3. Neodymium L 3-edge XANES spectra for (a) eudialyte and (b) selected standards. Cerium L 2-edges are visible in the pre-edge region for Nd (at 6168 eV for Ce3+ and 6179 eV for Ce4+). The black line indicates the main Nd L 3-edge at 6213 eV. Grey vertical lines labelled A (6215 eV), B (6250 eV) and C (6286 eV) mark the main features in the eudialyte spectra. Differences in relative heights of absorption edges reflect natural variations in Ce3+/Ce4+ and Ce/Nd ratios in the analysed minerals. Note the absence of Ce absorption in the synthetic phases and Nd in aqueous solution.
As for the standards, the eudialyte spectra show a sharp and narrow white line at the Nd L 3-edge (6213 eV) and small Ce L 2-edge absorption peaks in the pre-edge region. Two characteristic features are marked in the eudialyte Nd XANES, labelled B and C (Fig 3b). Minor variations in the position and relative height of these features, as well as variations in the relative height of the Ce3+L 2-edge peak (at 6168 eV) compared to the Nd3+L 3-edge peak (6215 eV), reflect variable REE concentrations in eudialyte between samples and localities (eudialyte compositions in Table S2). Absorption features for Ce4+ (c. 6179 eV) are generally absent in eudialyte, although a slight contribution of Ce4+ may be recognised in eudialyte of Norra Kärr.
Y K-edge EXAFS
The k 2-weighted EXAFS oscillations as a function of wavenumber (k–space) are demonstrated in Fig. 4 along with phase-shifted Fourier-transform functions for a selection of Y standards and the eudialyte samples. The EXAFS oscillations for all eudialyte samples are near-identical (black lines, Fig. 4c), suggesting an identical structural state for Y in the studied specimens. Accompanying Fourier-Transform functions (Fig 4d) demonstrate two distinct coordination spheres (shells) at a radial distance of ~2.3 Å and ~3.5 Å, suggesting the presence of scattering atoms at those distances and corresponding well to the structural model for the M1 site (Table 3). Also shown in Fig. 4 are best-fit results for the standards and samples (fits provided in Table S5), and refined parameters from which the fits are derived listed in Table 4. The refinement results are discussed further in the following sections.
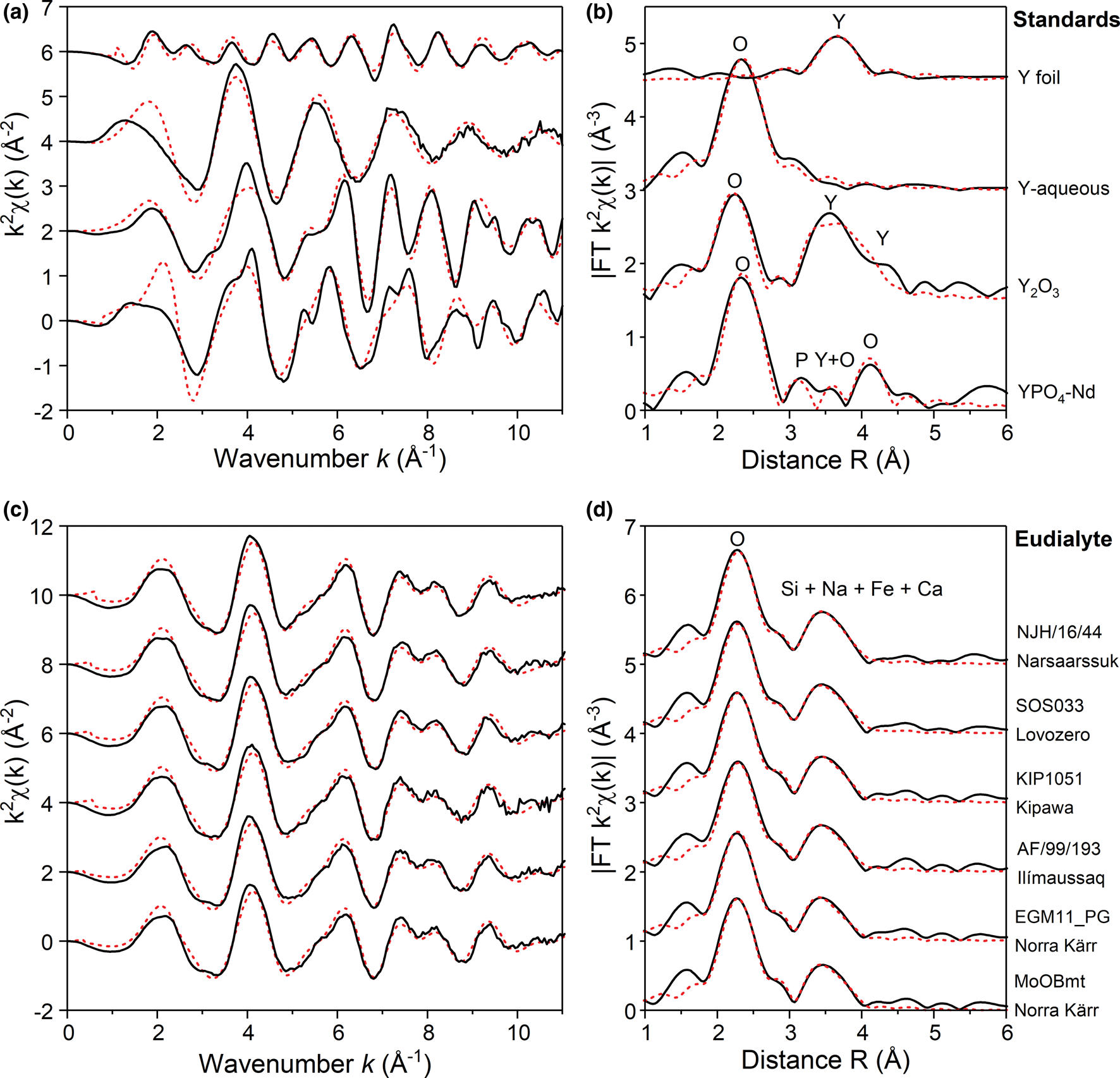
Fig. 4. K 2-weighted Y K-edge EXAFS oscillations and corresponding phase-shifted Fourier transforms (FT) for (a,b) selected Y standards and (c, d) eudialyte samples. Black lines represent experimental data, and the red dashed lines are least-square fits obtained in Artemis. Fitting parameters are given in Table 4 (standards) and Table 5 (eudialyte). Note the identical EXAFS patterns for eudialyte samples of different provenance.
Discussion
XANES
Normalised Y K-edge XANES for eudialyte and standards are shown and annotated in Fig. 2. The standards were selected to demonstrate a range of REE coordination states and point symmetries, such that potential variations in XANES features could be analysed qualitatively. Although limited Y K-edge XANES data are available in the literature, measured spectra for standards Y2O3, Y3+aqueous and YPO4 compare well to published spectra (Dura et al., Reference Dura, Boada, López de la Torre, Aquilanti, Rivera-Calzada, Leon and Chaboy2013; Tanaka et al., Reference Tanaka, Takahashi and Shimizu2008, Reference Tanaka, Takahashi and Shimizu2009). The standards and samples demonstrate variable absorption features, labelled A to D in Fig 2. Overall, phases in which REE are inferred to occupy higher point symmetry sites show more pronounced absorption features. We infer that sites with higher point symmetry favour the multiple resonances that generate XANES with more detailed fine structure, whereas low point symmetry sites give rise to broader XANES features. Another observation is the apparent shift of c. 10 eV in the position of feature D with changing coordination states (Fig 2b). The position of the peak at feature D for the EGM samples lies at 17,110 eV and is most comparable to the spectra for standards in which Y are inferred to occupy 6-coordinated sites (Fig. 2b,d). From this we tentatively infer that Y in EGM is 6-coordinated. However, none of the 6-coordinated standards display the characteristic double-peaked and broadened absorption edge observed for eudialyte (i.e. feature A at 17,053 eV and C at 17,061 eV). Hence, within our limited XANES dataset for Y-bearing minerals, the eudialyte XANES spectra are unique. If we disregard the anomalous spectrum for AF/99/193, which was collected using a small (3 µm × 3 µm) beam at I18, the consistency in the other eudialyte XANES spectra suggests that Y occupies the same structural state in all measured samples. We interpret the anomalous spectrum as the illumination of an inclusion or an alteration product within the crushed sample. This particular spectrum shows a characteristic shoulder at 17,065 eV and additional features at 17,099 and 17,115 eV, corresponding closely to the XANES features of zircon (Fig 2a). This may suggest the presence of 8-coordinated Y in microscopic zircon as an alteration product of eudialyte (van de Ven et al., Reference van de Ven, Borst, Davies, Hunt and Finch2019).
The Nd L 3-XANES show narrow white lines that are characteristic for L-edge absorption spectra (de Groot, Reference de Groot1995). Unlike the Y K-XANES, there is no systematic change with increasing coordination number in the Nd L 3-XANES (Fig 3b). Variations in the height of the pre-edge absorption features demonstrate natural variations in Ce3+/Ce4+ and Ce/Nd ratios. Our reference sample from the most oxidised environment is a hydrothermally altered zircon derived from a lateritic weathering profile on peralkaline rocks of the Ambohimirahavavy complex, Madagascar (Estrade et al., Reference Estrade, Salvi, Béziat, Rakotovao and Rakotondrazafy2014), which accordingly shows a stronger Ce4+ absorption feature at 6179 eV. Similar to the Y XANES, standards with higher point symmetries (‘yttrofluorite’, xenotime (YPO4–Nd), Nd-aqueous) show more pronounced Nd XANES features and narrower absorption peaks. The Nd XANES for zircon appears to be an exception to this rule, suggesting that a proportion of the larger LREE 3+, in contrast to HREE 3+, do not substitute on the high symmetry 8-fold Zr-site but on low symmetry interstitial sites (Friis et al., Reference Friis, Finch, Williams and Hanchar2010), metamict parts of the structure, or a combination of the two. The XANES spectra for Nd in aqueous solution (weak nitric acid) exhibit a particularly strong white line and additional features that are not observed in the other absorption spectra. From this we infer that Nd in aqueous solution occurs as highly ordered and symmetric Nd–OH complexes.
Overall, Nd XANES for eudialyte are similar to the XANES for those REE-standards in which the REE occupy low-symmetry sites, but with different coordination numbers from the inferred REE sites in eudialyte, in particular steenstrupine, A1, fluorapatite and allanite-(Ce), parisite-(Ce) and bastnäsite-(Ce). As such, the Nd L 3-edge appears to be insensitive to coordination number but rather reflects the point symmetry of the site. However, we infer that Nd, along with the other light REE, occupy both the 6-fold M1 site and the low symmetry 9-coordinated N4 site.
EXAFS refinements
Standards
Prior to modelling the Y K-EXAFS data to the complex structural models for eudialyte, we first fitted EXAFS spectra for selected Y standards (Y foil, Y oxide, YPO4 and Y in solution) to test the accuracy of our refinement procedures in Artemis.
The EXAFS spectrum for Y2O3 compares well to those reported by Lazdins and Kuzmin (Reference Lazdins and Kuzmin2015) and Tanaka et al. (Reference Tanaka, Takahashi and Shimizu2008) and is fitted to the cubic-Y2O3 structure from Santos et al. (Reference Santos, Strecker, Suzuki, Kycia, Silva and Silva2005), comprising three single-scattering paths of 6 O at 2.28 Å, 6 Y at 3.51 Å and 6 Y at 4 Å. When fixing the coordination numbers, fitted bond distances for the single-scattering paths are 2.271, 3.53 and 4.01 (± 0.01) Å, respectively (R factor = 0.035, Table 4). These are consistent with the structural model and within error of EXAFS parameters obtained by Tanaka et al. (Reference Tanaka, Takahashi and Shimizu2008).
Speciation studies of REE in aqueous solutions at ambient temperatures suggest that the light rare earths (La–Eu) favour a 9-fold hydration sphere, while the HREE (Gd–Lu, including Y) favour 8-fold hydration (Kanno and Hiraishi, Reference Kanno and Hiraishi1984; Díaz-Moreno et al., Reference Díaz-Moreno, Muñoz-Páez and Chaboy2000; Persson et al., Reference Persson, D'Angelo, De Panfilis, Sandström and Eriksson2008; Tanaka et al., Reference Tanaka, Takahashi and Shimizu2008; Finck et al., Reference Finck, Bouby, Dardenne and Yokosawa2017; Liu et al., Reference Liu, Etschmann, Migdisov, Boukhalfa, Testemale, Müller, Hazemann and Brugger2017), with average rare-earth to oxygen bond distances steadily decreasing for the light to the heavy REE. A single-shell fit for the EXAFS pattern (Fig. 4a,b) of Y3+ dissolved in weak nitric acid yields a best fit for 8.3 ± 0.4 oxygens (of surrounding water molecules) at 2.380 ± 0.005 Å (σ2 = 0.011 ± 0.002 Å–2, R factor = 0.009, Table 4). These distances are longer than those reported for aqueous Y3+ in previous EXAFS studies (2.354 ± 0.005 Å, Tanaka et al. (Reference Tanaka, Takahashi and Shimizu2008) and 2.353 ± 0.001 Å, Díaz-Moreno et al. (Reference Díaz-Moreno, Muñoz-Páez and Chaboy2000). However, the coordination number is consistent with the literature indicating Y in solution predominantly occurs in an 8-fold hydration sphere. The solutions studied by other authors were mostly neutral whereas our solution was acidic (0.3M HNO3, pH = 0.5) and we infer that protonation of the Nd complex has modified the bond distance.
The coordination sphere around Y in xenotime is 8-fold, similar to the coordination of REE in zircon. Four individual coordination shells are visible of the Fourier-transform function (Fig. 4b), corresponding to surrounding O, P and Y atoms within the 4.3 Å range. The spectrum was fitted to a structural model of YPO4 from Ni et al. (Reference Ni, Hughes and Mariano1995) over an adjusted k range of 2.5–10 Å–1 and R range of 1.25–4.2 Å to include all shells observed in the Fourier transform, yielding bond distances consistent with the model for all relevant scattering paths (R factor 0.047, Table 4).
The Fourier transform for the metallic Y foil reveals a single coordination shell around 3.6 Å, and an EXAFS signal that is somewhat attenuated (Fig. 4a,b). The coordination sphere of Y in metallic form is 12-fold, comprising 6 Y atoms at 3.56 Å and 6 Y at 3.65 Å in a hexagonal close-packed structure (Wyckoff, Reference Wyckoff1964). The spectrum is successfully fitted to the model with 12 Y (fixed CN) at an average distance of 3.59 ± 0.01 Å (σ2 = 0.011 Å–2, R factor = 0.015, k range = 2.5–9 Å–1, and R range = 2.5–4 Å), while floating the amplitude reduction factor to account for the attenuated signal (Table 4). More refinement details for this standard are described in Supplementary 1.
Eudialyte-group minerals
To determine the coordination sphere of Y in the structure of EGM, EXAFS spectra for each sample were fitted to structural models of the M1, N and Z sites as discussed in the introduction. Fitting was done in a step-wise manner by sequentially including more scattering paths (increasing the R range), while minimising the number of variables per fit to ensure a determinacy of >2. In the first step of the refinement, yttrium–oxygen bond distances, Debye–Waller factors and ΔE 0 were fitted for the first shell only, using a fixed amplitude reduction factor (S 02 = 1) and a reduced R range of 1–2.5 Å (k range = 2.5–10 Å–1). For the distorted octahedral M1 site, we provided a structural model with two oxygen paths, one to model the two shorter bonds (Y–O1a) and one to model the four longer bonds (Y–O2b) (Fig 1, Table 3). The first shell fits yield robust Y–O distances of 2.23 ± 0.01 Å and 2.30 ± 0.01 Å for the short and long Y–O paths, respectively (R factors of 0.010–0.016), indicating on average 3% shortening of the M1 cation–oxygen bond distances when Ca is replaced by Y.
In the second step, we extended the R range to 1–4 Å to include the second peak visible in the Fourier-transform function (Fig. 4d). The broad shape of this peak suggests the presence of next-nearest neighbour atoms at distances between 3.4 and 3.6 Å, consistent with the expected cluster of next-nearest neighbours for the M1 site (Fe, Ca, Na, O and Si; Table 3). Refinement of the scattering paths in this second shell is challenging due the variety of elements present at this radial distance to the M1 site (see Fig. 6, below), and hence the high number of variables required in the fitting procedure. However, an attempt was made to fit all relevant scattering paths within 3.7 Å distance of the absorbing Y atom. Over the fitted k- and R-range of 2.5–10 Å–1 and 1–4 Å, respectively, the number of independent variables (N) supported by the fit is 14.11. To ensure a determinacy above 2 (N/P), 7 parameters (P) could be fitted in the procedure. We reduced the number of fitted variables to <7 by fixing ΔE 0 to the value obtained from the first shell fit, and by using the same σ2 and ΔR for all oxygen paths. Fit parameters for σ2 and ΔR for the other paths were iteratively evaluated and amalgamated when giving similar values or high correlation factors between parameters.
The final two-shell fits yield R factors between 0.015 and 0.021 (Table 5). Results are shown in Fig. 4c and d. Isolated contributions for each scattering path are shown in Fig. 5 to illustrate the most important scatterers in the second coordination sphere; Si and Ca, respectively, belonging to 6 corner-linked [SiO4] tetrahedra and two edge-linked M1 sites (Fig. 6). The other scatterers; Fe, Na, and O (part of one edge-linked M2 site, and two side-linked N-sites, respectively) only make minor contributions to the overall EXAFS signal (Fig. 5). Accordingly, the Fe (or Mn) and Na path parameters are less well constrained, which is reflected in more variable bond distances, larger Debye–Waller factors and greater uncertainties (Table 5).

Fig. 5. Radial distribution function (phase-shifted k 2-weighted Fourier transforms) showing contributions of individual scattering paths to the final fit (black line) for eudialyte Y EXAFS refinements (as shown in Fig 4c,d) on the M1 site (in eudialyte s.s.). All single-scattering paths within 3.7 Å distance of the central atom in M1 (Table 3, Fig 6) are shown. Contributions of multiple-scattering are negligible and not considered in the refinements.

Fig. 6. Local structure for Y-occupied M site showing nearest neighbour polyhedra as probed by EXAFS. Projection along [110] showing half of a six-membered M16O36-ring. Anions (oxygen and chlorine) are not shown.
Table 5. Yttrium EXAFS refinements for the eudialyte-group minerals studied.

aFinal parameters are fitted over the k range 2.5–10 Å−1 and R range 1–4 Å
Given the complexity of the second coordination sphere, it is important to note that the quality of the refined parameters strongly depend on the structural model used, i.e. the assumed site and type of EGM in question. Because all samples are refined to a structural model for the M1 site in eudialyte s.s., compositional variations and associated changes in geometry and type of second-nearest neighbour scatterers among the analysed samples are unaccounted for. These would influence the type of scatterers present in the second coordination sphere, but not the first shell parameters for the geometry of the REE site. Refinements of the data for REE using structural models for the N4 and Z sites were unsuccessful.
We can summarise the Y K-edge X-ray absorption data for eudialyte-group minerals as follows: (1) we find an overall consistency in the morphology of the XANES and EXAFS for the measured eudialyte; (2) identified XANES features are most comparable to standards where REE occupy 6-coordinated sites; (3) Y–O bond distances of 2.24–2.3 Å are consistent with Y substitution for octahedral Ca on the M1 site; (4) Y substitution on the M1 site results in 3% shortening of M1 site bond lengths relative to Ca-occupied M1 bond lengths (corresponding to a 9% volumetric contraction); and (5) the second coordination sphere is successfully modelled with expected next-nearest neighbours (Si, Ca, Fe, Na and O) up to a radial distance of 3.6 Å around the M1 central atom.
Our results are consistent with Y (and by inference other heavy REE) dominantly substituting for Ca on the M1 site. The local geometry of sites surrounding the Y-occupied M1 site (half a six-membered ring of edge-shared M1 octahedra) as fitted to the EXAFS data is shown in Fig. 6.
Lattice strain REE partitioning models
The Y EXAFS refinements indicate that Y–O bond distances are ~0.08 Å (3%) shorter than average Ca–O bond lengths on the M1 site as inferred from XRD refinement. The ionic radius for Y3+ (0.9 Å) is ~10% smaller than divalent Ca2+ (1 Å) in octahedral coordination, and so the ~3% contraction in bond distances is a non-linear response to the substituent. Most structures accommodate minor and trace substituents by expanding or collapsing around the larger or smaller atom. Some structures (e.g. Ca site in calcite, Finch and Allison, Reference Finch and Allison2007), flex readily, expanding and contracting as elements are placed on the site to provide bond distances that match the sum of ionic radii for the substituent and oxygen. In such cases the local coordination is dictated by the size of the substituent. Conversely, structures may be rigid, where the geometry of the site is fixed and the substituent ion must comply with it. The ‘stiffness’ of a site is expressed by its Young's modulus (E, in GPa), i.e. rigid sites have large Young's moduli and those with lower values are flexible. Sites that flex readily will more easily accommodate elements of varying ionic radii. This concept is quantitatively expressed by the lattice strain model of Blundy and Wood (Reference Blundy and Wood1994), which allows the prediction of trace-element partitioning as a function of ionic radius and Young's modulus of the site. In this section we explore the lattice strain model for REE in eudialyte-group minerals in light of the EXAFS results.
Our EXAFS data do not provide evidence for Y on either the N, M2 or Z sites in the EGM structure, and instead indicate that Y (and by inference other similarly sized heavy REE) substitutes mainly for Ca on the octahedral M1 site. Substitution of 9-fold Y3+ (1.08 Å) for 9-fold Na+ (1.24 Å) on the N site may not be anticipated due to substantial differences in size and charge. Yet XRD refinements commonly infer the presence of light REE, as well as Sr, K, Ca and H2O on the N site, suggesting these sites are relatively flexible. Similarly, substitution of 6-fold Y3+ (0.9 Å) for 6-fold Zr4+ (0.72 Å) would be unlikely without posing considerable strain on the overall structure. Our failure to identify Y on the Z site suggests that the Z site is indeed too rigid to accommodate larger elements. This hints at substantial contrasts in the Young's moduli of the different sites in the eudialyte structure. Indeed, experimental data for cation–anion polyhedra in silicates and oxides demonstrate a linear increase of Young's moduli of a site with its charge to size ratio (the Z/d 3 ratio, where d is bond distance; Blundy and Wood, Reference Blundy and Wood2003; Hazen and Finger, Reference Hazen and Finger1979). On the basis of this relationship we can make a first order estimate of the Young's moduli for sites in the EGM structure by extrapolation from their Z/d 3 values (Fig. 5 in Blundy and Wood, Reference Blundy and Wood2003). Young's moduli estimated in this way for the N4, M1, M2 and Z sites in eudialyte s.s. occupied by IXNa+,VICa2+, IVFe2+ and VIZr4+, respectively, are 80, 200, 600 and 3000 GPa (Table 6).
Table 6. Site parameters and estimated Youngs moduli for lattice strain calculations.

a Mean bond distance from crystallographic data; bideal size of site r 0 based on crystallographic bond distance minus the ionic radius of oxygen (1.38); cYoung's Modulus (E) estimated from charge to size relations (Z/d 3) in Blundy and Wood (Reference Blundy and Wood2003); dreference for the crystallographic data.
Using the lattice strain equation (Blundy and Wood, Reference Blundy and Wood1994, modified after Brice, Reference Brice1975) we can calculate relative partition coefficients for a series of isovalent elements on a site ‘M’, as a function of ionic radii (r i):
 $$\displaystyle{{D_i} \over {D_{0\lpar M \rpar }^{n +}}} = exp\left\{ {\displaystyle{{-4{\rm \pi} {\rm N}_{\rm A}E_M^{n +} \left[ {\displaystyle{1 \over 2}r_0^{n +} {\lpar {r_i-r_{0\lpar M \rpar }^{n +}} \rpar }^2 + \displaystyle{1 \over 3}{\lpar {r_i-r_{0\lpar {M1} \rpar }^{n +}} \rpar }^3} \right]} \over {{\rm R}T}}} \right\}$$
$$\displaystyle{{D_i} \over {D_{0\lpar M \rpar }^{n +}}} = exp\left\{ {\displaystyle{{-4{\rm \pi} {\rm N}_{\rm A}E_M^{n +} \left[ {\displaystyle{1 \over 2}r_0^{n +} {\lpar {r_i-r_{0\lpar M \rpar }^{n +}} \rpar }^2 + \displaystyle{1 \over 3}{\lpar {r_i-r_{0\lpar {M1} \rpar }^{n +}} \rpar }^3} \right]} \over {{\rm R}T}}} \right\}$$where NA is the Avogadro's number, R the gas constant, T is temperature in Kelvin,  $r_{0\lpar M \rpar }^{n +} $ the zero strain radius of site M and
$r_{0\lpar M \rpar }^{n +} $ the zero strain radius of site M and  $E_M^{n +} $ its Young's modulus. As the difference in radius between the substituent and the site increases, relative partition coefficients decrease parabolically from a maximum value of 1 at
$E_M^{n +} $ its Young's modulus. As the difference in radius between the substituent and the site increases, relative partition coefficients decrease parabolically from a maximum value of 1 at  $r_{0\lpar M \rpar }^{n +} $. The tightness of the parabola (Onuma curve) relates to the Young's modulus, i.e.
$r_{0\lpar M \rpar }^{n +} $. The tightness of the parabola (Onuma curve) relates to the Young's modulus, i.e.  $E_M^{n +} $(Fig. 7). Note that equation 1 is rewritten to provide relative partition coefficients
$E_M^{n +} $(Fig. 7). Note that equation 1 is rewritten to provide relative partition coefficients $ \big( {{{D_i} \over {D_{0\lpar M \rpar }^{n +}}}} \big),$ rather than absolute partition coefficients (D i). This is because no absolute mineral-melt partitioning data are available for the eudialyte-group minerals, and hence
$ \big( {{{D_i} \over {D_{0\lpar M \rpar }^{n +}}}} \big),$ rather than absolute partition coefficients (D i). This is because no absolute mineral-melt partitioning data are available for the eudialyte-group minerals, and hence  $D_{0\lpar M \rpar }^{n +} $ is unconstrained. This absence of data is largely a consequence of poorly constrained melt compositions, P-T-X conditions and the absence of experimentally derived partitioning data for agpaitic systems. Nevertheless, the models provide a tool to predict partitioning behaviour of heavy and light REE onto the various sites in the EGM structure, and how this behaviour might change as a function of EGM crystal chemistry.
$D_{0\lpar M \rpar }^{n +} $ is unconstrained. This absence of data is largely a consequence of poorly constrained melt compositions, P-T-X conditions and the absence of experimentally derived partitioning data for agpaitic systems. Nevertheless, the models provide a tool to predict partitioning behaviour of heavy and light REE onto the various sites in the EGM structure, and how this behaviour might change as a function of EGM crystal chemistry.

Fig. 7. Schematic Onuma curves plotting relative partition coefficients (D i/D 0) against ionic radii for series of uni-, di- and trivalent cations onto site M 2+. The width of the parabola reflects the flexibility of the site, expressed by the Young's modulus (E), which increases with charge (see explanation in text). Vertical offsets reflect the electrostatic penalty incurred by the mismatch in charge between the substituent cation and the site, calculated from Wood and Blundy's (2001) electrostatic model. Horizontal offsets between the parabola reflect decreasing r 0 with increasing charge.
For heterovalent substitutions, in this case trivalent REE substituting for divalent, univalent or tetravalent cations, various electrostatic penalties are incurred by the mismatch in charge which need to be accounted for in the lattice strain models. As summarised in Wood and Blundy (Reference Wood, Blundy, Holland and Turekian2014), substituent charge (Z) considerably affects lattice strain partitioning curves, as D 0, r 0 and E are all found to vary with valence. Firstly, D 0 decreases with increasing charge offset, due to the additional work required to offset the imbalance (Wood and Blundy, Reference Wood and Blundy2001). For most minerals, the charge-dependence of D 0 is found to be near-parabolic, and can be quantified by the electrostatic model of Wood and Blundy (Reference Wood and Blundy2001):
 $$\displaystyle{{D_{0\lpar M \rpar }^{n +}} \over {D_{00\lpar M \rpar }}} = \exp \left\{ {\displaystyle{{-4{\rm \pi} {\rm N}_{\rm A}e_0^2 {\lpar {Z_n-Z_{0\lpar M \rpar }} \rpar }^2} \over {2{\rm \varepsilon R}T}}} \right\}$$
$$\displaystyle{{D_{0\lpar M \rpar }^{n +}} \over {D_{00\lpar M \rpar }}} = \exp \left\{ {\displaystyle{{-4{\rm \pi} {\rm N}_{\rm A}e_0^2 {\lpar {Z_n-Z_{0\lpar M \rpar }} \rpar }^2} \over {2{\rm \varepsilon R}T}}} \right\}$$where ɛ is charge on the electron. Following this equation, excess charge and deficit charge are penalised equally, such that  $D_0^{2 +} \gt D_0^{3 +} \approx D_0^{1 +}$ (Wood and Blundy, Reference Wood and Blundy2001). In our models, this amounts to an equal vertical offset in D i/D o for isovalent and trivalent elements that substitute onto a divalent site, as shown in the schematic diagram of Fig. 7.
$D_0^{2 +} \gt D_0^{3 +} \approx D_0^{1 +}$ (Wood and Blundy, Reference Wood and Blundy2001). In our models, this amounts to an equal vertical offset in D i/D o for isovalent and trivalent elements that substitute onto a divalent site, as shown in the schematic diagram of Fig. 7.
The second effect of charge is that the zero strain radius of a site decreases with increasing charge on the substituent element, i.e.  $r_{0\lpar M \rpar }^{1 +} \gt r_{0\lpar M \rpar }^{2 +} \gt r_{0\lpar M \rpar }^{3 +} $ (Wood and Blundy, Reference Wood, Blundy, Holland and Turekian2014). The extent to which r 0 decreases with increasing charge varies per mineral, and can be substantial (>0.1–0.2 Å per ΔZ) as observed for octahedral Ca sites in wollastonite (Law et al., Reference Law, Blundy, Wood and Ragnarsdottir2000) or relatively minor (<0.03 Å per ΔZ), as observed for Ca on the M2 site in diopside (Wood and Blundy, Reference Wood and Blundy1997). The magnitude of this offset depends on mineral composition, bulk properties, PT conditions, and a mineral's capacity to balance charge by coupled substitutions on nearest-neighbour sites. Given the complex solid-solution schemes and charge-balancing substitutions possible in EGM, we assume a relatively small charge dependence of r 0 for the considered sites. As such, we approach this charge effect by using Δr 0 values similar to that observed for the octahedral Ca-occupied M2 site in diopside by Wood and Blundy (Reference Wood and Blundy1997), as follows:
$r_{0\lpar M \rpar }^{1 +} \gt r_{0\lpar M \rpar }^{2 +} \gt r_{0\lpar M \rpar }^{3 +} $ (Wood and Blundy, Reference Wood, Blundy, Holland and Turekian2014). The extent to which r 0 decreases with increasing charge varies per mineral, and can be substantial (>0.1–0.2 Å per ΔZ) as observed for octahedral Ca sites in wollastonite (Law et al., Reference Law, Blundy, Wood and Ragnarsdottir2000) or relatively minor (<0.03 Å per ΔZ), as observed for Ca on the M2 site in diopside (Wood and Blundy, Reference Wood and Blundy1997). The magnitude of this offset depends on mineral composition, bulk properties, PT conditions, and a mineral's capacity to balance charge by coupled substitutions on nearest-neighbour sites. Given the complex solid-solution schemes and charge-balancing substitutions possible in EGM, we assume a relatively small charge dependence of r 0 for the considered sites. As such, we approach this charge effect by using Δr 0 values similar to that observed for the octahedral Ca-occupied M2 site in diopside by Wood and Blundy (Reference Wood and Blundy1997), as follows:  $r_0^{3 +} = r_0^{2 +} -0.01$Å and
$r_0^{3 +} = r_0^{2 +} -0.01$Å and  $r_0^{1 +} = r_0^{2 +} + 0.03$Å.
$r_0^{1 +} = r_0^{2 +} + 0.03$Å.
The third effect of charge on the substituent is that the effective Young's modulus of the site increases with increasing charge, i.e.  $E_M^{1 +} \lt E_M^{2 +} \lt E_M^{3 +} $. Again, we can use fig. 5 in Blundy and Wood (Reference Blundy and Wood2003) to estimate Young's moduli from the z/d 3 ratios for each isovalent series of elements. The resulting theoretical partitioning curves, which take into account effects of charge on D 0,r 0 and E (Fig. 7), are calculated using equation 1 and 2 and shown in Fig. 8. Elements are projected onto the isovalent partitioning curves for each of the considered sites. Young's moduli and site parameters used to calculate the curves are summarised in Table 6. The full calculation sheet is provided in Table S5.
$E_M^{1 +} \lt E_M^{2 +} \lt E_M^{3 +} $. Again, we can use fig. 5 in Blundy and Wood (Reference Blundy and Wood2003) to estimate Young's moduli from the z/d 3 ratios for each isovalent series of elements. The resulting theoretical partitioning curves, which take into account effects of charge on D 0,r 0 and E (Fig. 7), are calculated using equation 1 and 2 and shown in Fig. 8. Elements are projected onto the isovalent partitioning curves for each of the considered sites. Young's moduli and site parameters used to calculate the curves are summarised in Table 6. The full calculation sheet is provided in Table S5.

Fig. 8. Relative Onuma curves calculated for the N4, M1, M2 and Z sites in eudialyte s.s. (Johnsen and Grice, Reference Johnsen and Grice1999). D i/D 0 are calculated from the lattice strain model of Blundy and Wood (Reference Blundy and Wood1994) using r 0 values that correspond to the radii of the dominant cation on the site. Thick stippled lines indicate EXAFS- and XRD-determined site dimensions (bond distance minus 1.38 Å radius of oxygen, Table 6) that could be taken as alternative zero strain radii (r 0) for the sites. Note variations in the horizontal and vertical positions, as well as the tightness of the parabolas with valency, due to charge effects on E S, r 0 and D 0, as summarised in Fig. 7. Input parameters for each site are given in Table 6.
A moderate Young's modulus for the Ca-dominated M1 site allows it to accommodate a wide range of substituents whose 6-fold radii are close to Ca such as Y and the lanthanides (Shannon, Reference Shannon1976). The N site has an ideal charge of + 1 and is highly flexible, but the lanthanides are relatively small and trivalent, and so partitioning onto this site is generally unfavoured (Fig. 8). The charge offset between REE 3+ and Zr4+ is the same as that between REE 3+ and Ca2+, which we here assume is equally penalised in terms of the electrostatic strain imposed on the structure (fig. 7 in Blundy and Wood, Reference Blundy and Wood2003). However, the Young's modulus of the Z site would be large, making it relatively stiff and thus intolerant of any cation that does not match the size and charge of 6-fold Zr (Fig. 8).
The partitioning models allow us to extrapolate the partitioning behaviour of the other lanthanides and trace elements whose coordination states and bonding environment we have not directly measured (Fig. 8). In 6-fold coordination, the larger trivalent lanthanides, La3+ and Ce3+, are closer in radius to Ca2+ (Shannon, Reference Shannon1976) and hence it is reasonable to infer that they are most likely to substitute on the M1 site. The smallest of the lanthanides, Lu3+, has an ionic radius of 0.86 Å (Shannon, Reference Shannon1976), which is equally different from VICa2+ (1 Å) as from VIZr2+ (0.72 Å). However, because of the substantial differences in Young's moduli and zero strain radius between the M1 and Z site, the M1 site is more tolerant of the radius imbalance than the Z site. Hence on the basis of lattice strain theory, we infer that even the smallest lanthanides will most favourably substitute for Ca onto M1. Conversely, only the highly charged and much smaller Hf4+, Ti4+ and Nb5+ (0.71, 0.67, and 0.64 Å in 6-fold coordination) are likely to replace Zr on the Z site (Fig. 8). This is consistent with XRD-based site allocations for these elements and the existence of EGM species where Ti dominates the Z-site (alluaivite) (Johnsen and Grice, Reference Johnsen and Grice1999; Rastsvetaeva, Reference Rastsvetaeva2007; Pfaff et al., Reference Pfaff, Wenzel, Schilling, Marks and Markl2010).
Using structural parameters derived from XRD from other species of EGM, we can also predict changes in REE partitioning behaviour as a function of EGM crystal chemistry. In the oneillite subgroup, half the M1 octahedra are occupied by Mn or Fe (Table 1) creating an ordered structure of alternating larger Ca–M1 sites and smaller Mn–M1 sites. We can construct a lattice strain model for an oneillite-type EGM (Fig. 9) which partitions REE over the larger Ca and smaller Mn sites. Six-fold Mn (0.83 Å) is slightly smaller than Lu (0.86 Å), and so is much closer in radius to the heavy REE than Ca. Therefore, when the M1 site is structurally occupied by Mn, substitution by the heaviest REE would impose significantly less elastic strain on the lattice than substitution by light REE (Fig. 9). Therefore, EGM species of the oneillite subgroup are likely to show a significant increase in the partitioning of heavy over light REE than EGM of the eudialyte subgroup, where Ca occupies all six M1 octahedra. The REE profiles interpolated from our elastic strain model estimated partition coefficients for eudialyte s.s. and oneillite are shown in Fig. 10. On the basis of the partitioning curves in Fig. 8 eudialyte s.s. is expected to have a chondrite-normalised REE pattern that strongly favours light REE over heavy (particularly Ce–Pr–Nd), whereas oneillite gives a profile that favours the smallest HREE as well as the larger LREE, and thus becomes relatively flat across the whole REE series. The sinusoidal profile interpolated from an oneillite-type substitution model is closer to the relatively flat REE patterns observed in natural EGM (Fig. 10). Our EXAFS data are insufficient to allow refinement of the occupancy of the Ca site to explore the hypothesis that HREE such as Y are clustered with Mn in oneillite-type microdomains. Nevertheless we hypothesise that clustering of Mn and heavy REE on M1 subsites in eudialyte s.s. gives rise to relatively flat to HREE enriched REE profiles. We conclude that EGM crystal chemistry and symmetry, in particular that of Ca and Mn content of the M1 site, plays an important role in REE partitioning behaviour and the capacity of EGM to fractionate light and heavy REE in the melt or fluids from which they crystallise.

Fig. 9. Relative Onuma curves calculated for the N4, M1, M2 and Z sites in oneillite subgroup members, where M1 octahedral rings consist of two non-equivalent subsites (M1a and b) which are occupied by Ca, Na, Mn, or Fe (Table 1). The example shown is for oneillite, Mn-raslakite and (Ca–Mn)-ordered eudialyte, where one of the M1 subsites is predominantly occupied by Mn, and the other by Ca, as in eudialyte s.s. For further explanation of diagram see the caption of Fig. 8.

Fig. 10. Projected chondrite-normalised REE patterns based on lattice strain partitioning models for eudialyte s.s. and oneillite-subgroup variations on the M1 site, as shown in Fig. 8 and 9. Note that melt compositions and absolute partitioning coefficients are unknown, and so the patterns are purely theoretical. Typical REE patterns for eudialyte-group minerals observed in nature are slightly LREE enriched with flat HREE patterns, with or without Eu anomalies. Europium anomalies are neglected in projected patterns, and depend on parental melt signatures (Schilling et al., Reference Schilling, Wu, McCammon, Wenzel, Marks, Pfaff, Jacob and Markl2011).
Summary and conclusions
We studied the substitution of rare earth elements in eudialyte-group minerals (EGM) using X-ray absorption spectroscopy. Yttrium K-edge and Nd L 3-edge XANES and EXAFS were collected for EGM from Ilímaussaq, Narsaarsuk, Norra Kärr, Kipawa and Lovozero and compared to absorption spectra for REE-bearing silicates, fluorides, phosphates and solutions. The EGM spectra exhibit reproducible XANES for Nd, as well as for Y, indicating consistent site occupancies for light REE and for heavy REE in EGM of varying composition and provenance. Yttrium K-edge XANES for EGM are unlike any of the measured standards and thus suggest a unique structural configuration. However, the EGM Y K-XANES share a characteristic feature (i.e. broad peak at 17,110 eV) with the standards in which the REE are inferred to occupy 6-fold coordinated sites. The Nd L 3-XANES for EGM are similar to those measured for the standards, and lack unique features that reflect the coordination state of Nd.
Quantitative refinement of the Y K-EXAFS suggests that yttrium dominantly substitutes for calcium on the crystallographic M1 site. Single-shell refinements yield optimal fits for Y in 6-fold coordination with Y–O bond distances between 2.24–2.32 Å. These bond distances correspond to a 3% contraction relative to ideal bond distances (XRD-determined, r o) in the Ca2+-dominant M1 site. The second coordination sphere in the EXAFS spectrum is successfully fitted to 2 Na, 1 Fe, 6 Si, 2 Ca and 4 O, present at an average distance of 3.5 Å to the M1 site. Our XAS refinements exclude preferential substitution of the smaller heavy REE onto the smaller octahedral Z site (typical Zr–O distances of 2.08 Å), as well as the larger polyhedral N site (average Na–O distances of 2.54 Å). We support our results using theoretical partitioning diagrams (Onuma curves) calculated using lattice strain theory on the basis of the ideal charge and size of the sites. These models predict that even the smallest lanthanides (HREE) are favoured on the octahedral M1 site, and that the relative partition coefficients of the heavy REE increase significantly relative to the light REE when Mn or Fe occupy a significant proportion of the M1 octahedral rings (e.g. in the Ca-deficient oneillite subgroup). Our data indicate that the relatively flat to HREE-enriched profiles that make eudialyte an attractive exploration target are not the result of heavy and light REE partitioning onto different structural sites. Rather we infer that ordering in M1 or coupling of HREE with smaller ions such as Mn produces the characteristically flat REE patterns observed in natural EGM.
Acknowledgements
This work was carried out under the NERC-funded SOS RARE consortium [grant NE/M010856/1 to AMB, NJH and AF]. We thank Diamond Light Source and KIT Karlsruhe Light Source for beam time at I18 [grants SP14793 and SP15903] and SUL-X, respectively. PG was funded by the People Programme (Marie Curie Actions) in the EU Seventh Framework Programme (FP7/2007-2013), REA grant agreement no. 609405 (COFUNDPostdocDTU), and wishes to dedicate this contribution to the memory of Dr Takeshi Kasama, his late mentor at the Center for Electron Nanoscopy. We thank Chris Hayward for assistance with EMPA analyses, Martin Smith, Peter Nason and Athanasios Godelitsas for assistance at I18 and SUL-X and Charlie Beard for discussions on lattice strain models. We thank the Geological Survey of Denmark and Greenland, Lynn Boatner (ORNL, USA), National Museum Scotland, the Hunterian Museum and SOSRARE partners for providing samples and standards. We thank three anonymous reviewers for constructive reviews and Roger Mitchell and Helen Kerbey for editorial handling.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1180/mgm.2019.50


















