Article contents
Vittinkiite, MnMn4[Si5O15], a member of the rhodonite group with a long history: definition as a mineral species
Published online by Cambridge University Press: 25 September 2020
Abstract
The rhodonite-group mineral with the idealised, end-member formula MnMn4[Si5O15] and the crystal chemical formula VIIM(5)MnVIM(1–3)Mn3VIIM(4)Mn[Si5O15] (Roman numerals indicate coordination numbers) is defined as a valid mineral species named vittinkiite after the type locality Vittinki (Vittinge) mines, Isokyrö, Western and Inner Finland Region, Finland. Vittinkiite is an isostructural analogue of rhodonite, ideally CaMn4[Si5O15], with Mn2+ > Ca at the M(5) site. Besides Vittinki, vitiinkiite was found in more than a dozen rhodonite deposits worldwide, however, it is significantly less common in comparison with rhodonite. The mineral typically forms pink to light pink massive, granular aggregates and is associated with quartz, rhodonite, tephroite, pyroxmangite and Mn oxides. Vittinkiite is optically biaxial (+), with α = 1.725(4), β = 1.733(4), γ = 1.745(5) and 2Vmeas = 75(10)° (589 nm). The chemical composition of the holotype (wt.%, electron microprobe) is: MgO 0.52, CaO, 0.93, MnO 51.82, FeO 1.26, ZnO 0.11, SiO2 46.48, total 101.12. The empirical formula calculated based on 15 O apfu is Mn4.71Ca0.11Fe0.11Mg0.08Zn0.01Si4.99O15. Vittinkiite is triclinic, space group P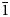 $\bar{1}$, with a = 6.6980(3), b = 7.6203(3), c = 11.8473(5) Å, α = 105.663(3), β = 92.400(3), γ = 94.309(3)°, V = 579.38(7) Å3 and Z = 2. The crystal structure is solved on a single crystal to R1 = 3.85%. Polymorphism of MnSiO3 (rhodonite-, pyroxmangite-, garnet- and clinopyroxene-type manganese metasilicates) is discussed, as well as the relationship between vittinkiite and pyroxmangite, ideally Mn7[Si7O21], and the application of infrared spectroscopy for the identification of manganese pyroxenoids.
$\bar{1}$, with a = 6.6980(3), b = 7.6203(3), c = 11.8473(5) Å, α = 105.663(3), β = 92.400(3), γ = 94.309(3)°, V = 579.38(7) Å3 and Z = 2. The crystal structure is solved on a single crystal to R1 = 3.85%. Polymorphism of MnSiO3 (rhodonite-, pyroxmangite-, garnet- and clinopyroxene-type manganese metasilicates) is discussed, as well as the relationship between vittinkiite and pyroxmangite, ideally Mn7[Si7O21], and the application of infrared spectroscopy for the identification of manganese pyroxenoids.
Keywords
- Type
- Article
- Information
- Copyright
- Copyright © The Author(s), 2020. Published by Cambridge University Press on behalf of The Mineralogical Society of Great Britain and Ireland
Footnotes
Associate Editor: Oleg I Siidra
References
- 1
- Cited by


