The traditional model of scientific discovery goes as such: Scientists plug away in laboratories, making fundamental discoveries and crafting theories to study and understand the physical world. Engineers then take over, advancing that fundamental knowledge and putting it to work by inventing practical devices.
But often the path is not that well defined or linear. Science and technology are symbiotic. Discovery can go both ways. The most effective and efficient advances often follow a cyclical path where science and technology feed each other. And engineering can many times lead to discoveries, with scientists gaining insights from devices and applications.
The steam engine, for instance, was developed before the elucidation of thermodynamic principles. Alexander Graham Bell, an engineer, invented the telephone armed with only a rudimentary knowledge of electricity and signal transmission. From his namesake, Bell Labs, came one of the modern world’s most important innovations and a prominent example of scientists and engineers working together: the discovery of the transistor effect and the technological invention of the transistor itself.
And sometimes breakthroughs come about from engineering research leading to, and even contradicting, established science. The discovery of the bright blue gallium nitride light-emitting diode (LED) is the perfect example. Isamu Akasaki, Hiroshi Amano, and Shuji Nakamura developed the blue LED in the early 1990s after others had given up, doggedly pursuing technological research despite skepticism, discouragement, and a prevailing scientific view that conflicted with their findings. “They were flying in the face of conventional wisdom,” said journalist Bob Johnstone, who recently wrote the book, LED: A History of the Future of Lighting.
The impact of blue LEDs cannot be exaggerated enough. Without it, we would not have the bright white LED lamps that have upended the way we light our world. Until its development, red and green LEDs had been around for three decades, used in indicator lamps and numeric displays. Today, high-efficiency, long-lasting white LED lamps light up buildings, streets, and TV screens.
As the Nobel Foundation said when they awarded the researchers the 2014 Nobel Prize in Physics, “They succeeded where everyone else had failed. Their inventions were revolutionary. Incandescent light bulbs lit the 20th century; the 21st century will be lit by LED lamps.”
Solid-state lighting has staggering implications for the world’s energy and resource use, since artificial lighting consumes 16% of the world’s electricity. The US Department of Energy projects that in 2035, LEDs will save about 490 TWh per year in the United States. That is as much as the output of 30 1 GW-scale power plants and would save US consumers USD$30 billion per year on electricity cost. For the whole world, the energy savings would be multiplied four times. LED lamps also save resources and labor because they typically last up to 100,000 hours, compared to 1000 hours for incandescent bulbs and 10,000 hours for fluorescent lights.
“The rapid adoption of LEDs in lighting marks one of the fastest technology shifts in human history,” global investment bank Goldman Sachs stated in a 2016 report, calling the LED industry “the top energy disrupter among green energy industries.”
Visible LEDs were born in 1962 when Nick Holonyak at the University of Illinois invented the first red LED; George Craford, Hewlett-Packard, followed a decade later with the invention of the yellow LED. Pioneering work on blue LEDs had also begun in the early 1960s at Stanford University and at television company RCA. A young RCA researcher named Herbert Paul Maruska managed to coax dim blue light from magnesium-doped gallium nitride (GaN) in 1972, creating the first blue LED.
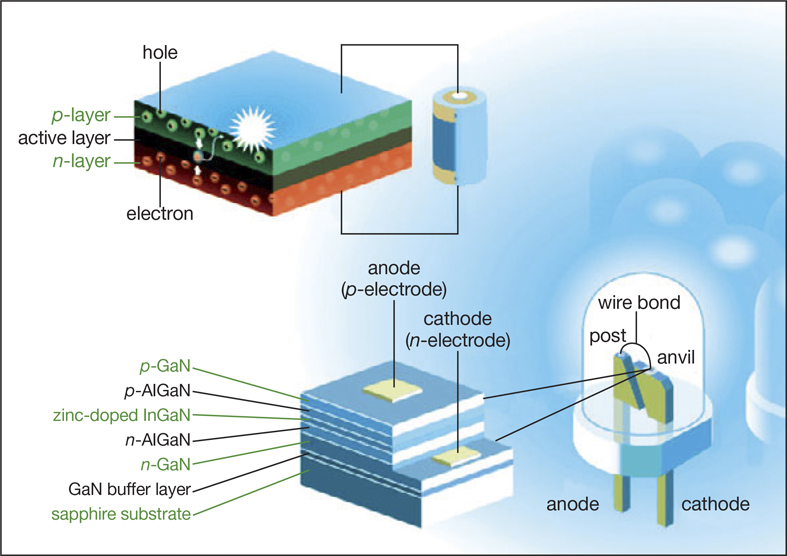
But blue LEDs as bright as their red and green cousins were still a seemingly insurmountable challenge for researchers. There were two big obstacles. One was the lack of a good substrate to grow GaN on. The other was the lack of a process to make efficient p-type GaN that was rich in positively charged holes: LEDs are made by placing electron-rich n-type and hole-rich p-type semiconductors next to each other.
By the 1980s, after years of trying, most scientists had given up on making blue LEDs from GaN. The first commercial blue LED, introduced in 1989 by Cree, Inc., was based on silicon carbide and had an efficiency of 0.03%. But Akasaki, Amano, and Nakamura were determined to work on GaN, despite others telling them that what they were attempting to do was impossible, and defied everything they understood.
The first big breakthrough for blue LEDs was a buffer layer for growing GaN. Sapphire is the best-known substrate for growing GaN, but the two materials’ crystal lattices do not match, leading to 10 billion defects per square centimeter. In the mid-1980s, Akasaki and Amano, working at Nagoya University, realized that they “needed a handshake layer between the gallium nitride and sapphire,” said Jung Han, a professor of electrical engineering at Yale University. “They found that when they made an aluminum nitride buffer layer on sapphire followed by gallium nitride, the crystal quality improved drastically.”
Nakamura, competing with Akasaki and Amano, and working alone and with minimal resources at Nichia Corporation, turned to GaN as a buffer layer. He grew it at low temperature in a home-built vapor-phase epitaxy reactor, and in 1991 was able to achieve a smooth surface with better electrical properties. “One of the reasons he was able to do it was precisely because he lacked resources,” said Johnstone. “He had years of experience using conventional technologies. He knew intricately how the epitaxy machine worked and was able to modify the machine to do exactly what he wanted it to do.”
In other words, this was pure engineering research. “Nobody understood why or how this worked until 10 years later in the mid- to late-90s,” said Han. “Then material scientists started to reconstruct or understand why this magic process works.” They recognized that atoms deposited at a low temperature nucleate and conform crystallographically to the underlying sapphire layer. And then, as the crystal grains are subsequently grown at a higher temperature, the ones that ware best aligned to sapphire grow faster at the expense of neighboring grains.
The blue LED is the key to practical LED lighting. © Johan Jarnestad, Royal Swedish Academy of Sciences.
The second breakthrough was the accidental discovery of p-type GaN. Zinc or magnesium would be the best p-type dopants for GaN, but they did not create holes in the material as expected. Akasaki and Amano realized during a microscopy imaging experiment that blasting doped material with an electron beam made it radiate blue light. This was a crucial discovery, but the method was too slow for manufacturing.
A few years later, Nakamura, who had suspected that heat turned the material p-type, had a major breakthrough. He found that heating a Mg-doped sample in a furnace created high-quality p-type GaN. He also explained the science behind the technological discovery. The culprit turned out to be hydrogen. Zinc or magnesium dopant atoms go into GaN coupled with hydrogen, which shields the dopants from accepting electrons and allowing the GaN to become p-type. Heat drives out the hydrogen, activating the acceptors.
The researchers now had what it took to make blue LEDs. Yet conventional wisdom stated that bright emission would be impossible from GaN because of its high density of defects, which trap electrons and holes and quench light by preventing their interaction.
But in 1994, Nakamura started reporting blue InGaN LEDs with higher and higher efficiencies. “His initial demonstration was so compelling that the entire world jumped on the bandwagon,” said Han. “The brightness was amazing compared to the previous LEDs made with silicon carbide. They were blindingly bright.”
Yet, for many years, scientists did not understand why. It took 10 years to unravel the mechanism. The commonly accepted theory is that InGaN alloys tend to separate into InN and GaN rich clusters, which impede the motion of electrons and holes and prevent them from being trapped by defects.
Nakamura’s bright blue LEDs were the beginnings of a lighting revolution. They have been mixed with yellow, red, and green LEDs to make white LED lamps.
The development of GaN blue LEDs shows how researchers sometimes have to step out of the bounds of prevailing scientific understanding for technological breakthroughs. It showcases the importance of engineering research that can precede the scientific understanding. And it highlights how scientific and engineering research can go hand in hand.
Often, however, a boundary is imposed between science and engineering research. Government funding decisions often separate and categorize research as one or the other. In industry, academia, and national laboratories, basic and applied research are compartmentalized, and there is a growing separation between the two.
Such dichotomy is not sustainable or beneficial. “We need to support both science and engineering research and bring them much closer together,” said Colin Humphreys, a professor of materials science and metallurgy at the University of Cambridge. Collaborative research is especially needed to meet today’s complicated challenges in knowledge domains such as energy and the life sciences.
Humphreys points to the discovery of gravitational waves as an example of how science and engineering research are symbiotic. “There is so much news about the scientific discovery of gravitational waves,” he said. “But what people miss is that the success is also due to very good engineering. These scientists could do their experiments because of the fantastic engineering.”
“Engineering is a bridge that connects science with society,” said Han. “When we do scientific research, it would be very useful to have awareness, knowledge, and perspective of possible applications. We should make sure we don’t lose sight of that.”


