Published online by Cambridge University Press: 30 July 2018
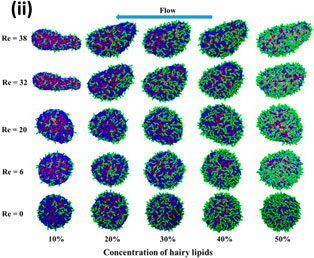
The flow of particles through confined volumes has appeared under different contexts in nature and technology. Some examples include the flow of red blood cells or drug delivery vehicles through capillaries, or surfactant-based particles in nano- or microfluidic cells. The molecular composition of the particles along with external conditions and the characteristics of the confined volume impact the response of the particle to flow. This review focuses on the problem of phospholipid vesicles constrained to flowing in channels. The review examines how experimental and computational approaches have been harnessed to study the response of these particles to the flow.
Equally contributing co-authors.