No CrossRef data available.
Article contents
Correlation Between Thermodynamic and Kinetic Properties of Glass-Forming Liquids
Published online by Cambridge University Press: 01 February 2011
Abstract
Correlations between three characteristic temperatures: glass transition, Tg , Kauzmann, Tk , and Vogel-Fulcher-Tammann, To , were identified from the analysis of more than 60 metallic and non-metallic glass-forming materials. It was found that Tg ≥ Tk ≥ To and Tk is the geometric mean of Tg and To 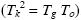 . The relation Tk ≥ To indicates that the excess total entropy of a super-cooled liquid ΔS approaches zero at a higher temperature than the configurational entropy ΔSconf , and such behavior was explained by the stronger temperature dependence of the excess vibrational entropy of the liquid, ΔSvib , than that of the corresponding glass,
. The relation Tk ≥ To indicates that the excess total entropy of a super-cooled liquid ΔS approaches zero at a higher temperature than the configurational entropy ΔSconf , and such behavior was explained by the stronger temperature dependence of the excess vibrational entropy of the liquid, ΔSvib , than that of the corresponding glass, 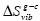 . A relationship between the fragility index m, reduced excess heat capacity ΔCp (Tg )/Sm , and reduced glass transition temperature, Trg , was identified using the found correlation between the characteristic temperatures.
. A relationship between the fragility index m, reduced excess heat capacity ΔCp (Tg )/Sm , and reduced glass transition temperature, Trg , was identified using the found correlation between the characteristic temperatures.
Information
- Type
- Research Article
- Information
- MRS Online Proceedings Library (OPL) , Volume 1048: Symposium Z – Bulk Metallic Glasses–2007 , 2007 , 1048-Z02-10
- Copyright
- Copyright © Materials Research Society 2008

