No CrossRef data available.
Article contents
Dilute Solution of H in bcc Ti35Cr65-xVx Alloys
Published online by Cambridge University Press: 01 February 2011
Abstract
The solubility of H in the solid solution phase of the Ti35Cr47V18 alloy has been investigated at high and low temperature. The Sieverts law has been found to hold for 808 K≤T≤1204 K and fail for 302 K≤T≤335 K. For H contents nH ≤0.07 (nH=H/Me) the partial molar enthalpy and partial non-configurational entropy at high temperature are:
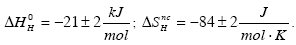
On heating the material under H2 gas pressures lower than 0.1 MPa H absorption only occurs above 700 K. Below this temperature, surface oxide films prevent H uptake. Thermal decomposition spectroscopy of the α phase carried out for nH≤0.24 exhibits a single peak whose temperature increases with decreasing nH owing to selective occupancy by H of energetically non-degenerate interstitial sites.
Keywords
Information
- Type
- Research Article
- Information
- Copyright
- Copyright © Materials Research Society 2008

