Introduction
Viewing sharks in their natural setting is a popular tourism activity (Gallagher & Hammerschlag, Reference Gallagher and Hammerschlag2011) and the income accrued creates an incentive to manage these charismatic species as a non-consumptive resource (Brunnschweiler, Reference Brunnschweiler2010; Clua et al., Reference Clua, Buray, Legendre, Mourier and Planes2011; Vianna et al., Reference Vianna, Meekan, Pannell, Marsh and Meeuwig2012). However, studies on elasmobranchs have documented situations in which marine tourism has negative effects, including behavioural changes and increased energetic costs (Pierce et al., Reference Pierce, Mendez-Jimenez, Collins, Rosero-Caicedo and Monadjem2010; Fitzpatrick et al., Reference Fitzpatrick, Abrantes, Seymour and Barnett2011). Improved understanding of the actual or potential effects of tourism interactions is important for mitigating or avoiding longer-term effects and ultimately safeguarding employment and the tourism infrastructure.
The whale shark Rhincodon typus is the largest fish and an iconic species for tourism (Gallagher & Hammerschlag, Reference Gallagher and Hammerschlag2011). Commercial whale shark interaction tours began in 1993 in Western Australia after a seasonal aggregation of the species was discovered within the Ningaloo Marine Park (Davis et al., Reference Davis, Banks, Birtles, Valentine and Cuthill1997). In 2006 the whale shark tourism industry at this site was valued at AUD 6 million annually (Catlin et al., Reference Catlin, Jones, Norman and Wood2010). Whale shark tourism industries have now developed at several locations in all three tropical oceans and are mostly based on the presence of predictable seasonal feeding aggregations of sharks exploiting ephemeral bursts in local productivity, such as mass fish or coral spawning events (Taylor, Reference Taylor1996; de la Parra Venegas et al., Reference de la Parra Venagas, Hueter, Gonzales Cano, Tyminski, Gregorio Remolina and Maslanka2011). The global revenue from whale shark tourism was provisionally estimated to be USD 42 million in 2007 (Graham, Reference Graham2007).
Whale sharks are categorized as Vulnerable on the IUCN Red List (Norman, Reference Norman2005). Although the economic value or potential of whale shark tourism has helped to justify legal protection for the species in some countries, concerns have also been raised that specialist tourism industries could negatively affect the sharks. Sightings of whale sharks at Gladden Spit in Belize declined during 1998–2003 (Graham & Roberts, Reference Graham and Roberts2007) and, based on anecdotal reports from guides, remained low at least until 2007 (Graham, Reference Graham2007). Graham (Reference Graham2007) suggested that the rapid increase in diver numbers at this site may have led to disturbance of snapper spawning behaviour (the main driver of whale shark presence), and the whale sharks themselves, although a dedicated study on disturbance by divers did not identify a direct effect on the sharks (Heyman et al., Reference Heyman, Carr and Lobel2010). Propeller injuries from small boats have also been observed on whale sharks at several aggregation sites (Rowat et al., Reference Rowat, Meekan, Engelhardt, Pardigon and Vely2007; Speed et al., Reference Speed, Meekan, Rowat, Pierce, Marshall and Bradshaw2008). Studies of the short-term behavioural responses of sharks to tourists and boats at Ningaloo Reef (Norman, Reference Norman1999), Donsol in the Philippines (Quiros, Reference Quiros2007) and Tofo Beach in Mozambique (Pierce et al., Reference Pierce, Mendez-Jimenez, Collins, Rosero-Caicedo and Monadjem2010) have revealed that sharks routinely display avoidance behaviours, including banking, eye-rolling, fast swimming and diving, in response to close approaches by swimmers or boats.
The ecological, social and economic sustainability of whale shark tourism at Ningaloo Reef was reviewed by Mau (Reference Mau2008). Although long-term empirical data on whale shark behaviour in the area were not available, the industry was judged to be ecologically sustainable. This was based on the lack of observed interruption of feeding behaviour, which may take place largely at night or at least outside tourist interaction times (Taylor, Reference Taylor2007), the regular re-sightings of philopatric sharks (Holmberg et al., Reference Holmberg, Norman and Arzoumanian2008, Reference Holmberg, Norman and Arzoumanian2009), and the lack of reproductive behaviour observed in this juvenile male-biased population (Meekan et al., Reference Meekan, Bradshaw, Press, McLean, Richards, Quasnichka and Taylor2006; Norman & Stevens, Reference Norman and Stevens2007). However, concerns were raised about the potential for injury from boat strikes (Speed et al., Reference Speed, Meekan, Rowat, Pierce, Marshall and Bradshaw2008) and an apparent decline in mean size over time (Bradshaw et al., Reference Bradshaw, Fitzpatrick, Steinberg, Brook and Meekan2008). Aside from Ningaloo Reef, where the sharks have been relatively well studied, the longer-term sustainability of whale shark tourism has not been considered explicitly.
Here we examine the tourism industry at Tofo Beach (Praia do Tofo) in Mozambique, an international hotspot for whale shark encounters (Pierce et al., Reference Pierce, Mendez-Jimenez, Collins, Rosero-Caicedo and Monadjem2010). Whale shark interactions are a key attraction for international divers visiting the country but a lack of official management has raised questions about the sustainability of the industry (Pierce et al., Reference Pierce, Mendez-Jimenez, Collins, Rosero-Caicedo and Monadjem2010; Tibiriçá et al., Reference Tibiriçá, Birtles, Valentine and Miller2011), particularly after a significant decline in sightings during 2005–2011 (Rohner et al., Reference Rohner, Pierce, Marshall, Weeks, Bennett and Richardson2013). Previous work at Tofo Beach has examined how interactions can be managed to minimize the potential for short-term negative effects on the sharks (Pierce et al., Reference Pierce, Mendez-Jimenez, Collins, Rosero-Caicedo and Monadjem2010). Here we extend that dataset to evaluate whether longitudinal encounter data reveal evidence of an increasing frequency of avoidance behaviours overall and whether individual-based analyses show evidence of changing avoidance behaviours over time. We consider the implications of these data and evaluate the longer-term implications for the ecology of whale sharks at this site.
Methods
The village of Tofo Beach is situated in the Inhambane province of Mozambique, c. 400 km north-east of the capital city, Maputo (Fig. 1). A full site overview and description of the commercial whale shark tourism industry in Tofo are provided by Pierce et al. (Reference Pierce, Mendez-Jimenez, Collins, Rosero-Caicedo and Monadjem2010). Tourism operators offer daily 2-hour snorkelling trips that aim to locate whale sharks and other marine megafauna. Vessels typically survey a 6 km stretch of coastline south of Tofo, between the surf line and c. 1,000 m from the shore, in waters c. 5–30 m deep. Sharks are located through visual inspection of the water surface, where it is often possible to spot their dark silhouettes or exposed fins. The boat is then positioned in relation to the shark's direction of travel and clients enter the water to interact with the shark.

Fig. 1 Tofo Beach, Mozambique. The rectangle on the inset shows the location of the main map in south-east Africa.
We collected data during January 2008–June 2010, except during July and August 2008 when no sampling trips took place. Upon locating a whale shark, observers entered the water alongside clients and recorded the total number of swimmers and environmental characteristics, including weather conditions (categorized as sunny, slightly overcast, overcast or raining), Beaufort sea state and underwater visibility. The total length of the shark was estimated visually (Rohner et al., Reference Rohner, Richardson, Marshall, Weeks and Pierce2011) and the sex identified by the presence or absence of claspers on the pelvic fins. The presence and location of any injuries or scars were noted and categorized post hoc as either major or minor (Speed et al., Reference Speed, Meekan, Rowat, Pierce, Marshall and Bradshaw2008). A basic ethogram of each shark's behaviour in the presence of swimmers was produced to record slow swimming, equating to normal behaviour; fast swimming, where there was an obvious increase in the shark's tail-beat frequency; diving, where the shark dived away from the surface; banking, where the shark rolled its back towards swimmers; changing direction, where the shark altered its direction of swimming in the presence of swimmers; and any other obvious avoidance behaviours, such as the violent shudder reported by Quiros (Reference Quiros2007). Observations of feeding behaviour were also noted when they occurred. The total encounter duration, defined as the time between the first swimmer entering the water and the last swimmer returning to the boat, was recorded (in minutes) following each interaction. When possible, standardized identification photographs of both the left and right flanks of each shark were taken and uploaded to the global Wildbook for Whale Sharks photo-identification library (Arzoumanian et al., Reference Arzoumanian, Holmberg and Norman2005; Marshall & Pierce, Reference Marshall and Pierce2012). Each of these encounters was then assigned to a new or previously identified shark in the library.
It was difficult to establish whether behaviours exhibited by whale sharks in the presence of swimmers would also occur in a natural, undisturbed setting. Within-effect comparisons (i.e. behaviour in the presence of swimmers) were therefore employed instead of attempting to determine cause-and-effect relationships. This approach is useful in cases where there are no baseline or control data, and focuses on assessing behavioural responses under gradations of the effect (Bejder & Samuels, Reference Bejder, Samuels, Gales, Hindell and Kirkwood2003). Fast swimming, diving, banking and changing direction are typically classified as avoidance behaviours in whale sharks (Norman, Reference Norman1999; Quiros, Reference Quiros2007; Pierce et al., Reference Pierce, Mendez-Jimenez, Collins, Rosero-Caicedo and Monadjem2010). When these or other obvious avoidance behaviours were observed during an interaction they were each assigned a score of one. The total score at the end of each encounter was thus equal to the total number of avoidance behaviours observed. All statistical analyses were carried out using R v. 2.11.0 (R Development Core Team, 2012), with MASS and nlme.
The relationship between avoidance score and encounter duration was tested using a generalized linear mixed model approach. Encounter duration, log transformed to ensure that error structure was normally distributed, was used as the response variable. Avoidance score was used as the explanatory variable and shark identification was added as a random effect to avoid pseudo replication. Given the significant relationship between avoidance score and encounter duration (see results; Pierce et al., Reference Pierce, Mendez-Jimenez, Collins, Rosero-Caicedo and Monadjem2010), encounter duration was used in all further analyses as a proxy for avoidance. A further model was fitted to determine which variables significantly affected encounter duration. Encounter duration was entered as the response variable and tested against nine main effect explanatory variables: whale shark variables (size, sex, presence/absence of scarring or feeding behaviour), environmental variables (weather, sea state (0–4) and underwater visibility), number of swimmers present during an encounter, and the Julian day (Day 1 = 9 January 2008). We tested second-order interactions between all combinations of whale shark variables; all combinations of environmental variables; size, sex and scarring and all environmental variables; and between the number of swimmers and underwater visibility. Year and month of encounter were entered as nested random effects. Only encounters in which shark identity had been confirmed were used in the analysis; a single interaction was randomly selected from those individuals for which multiple encounters had been recorded. We used a generalized linear model to test for a significant relationship between the amount of scarring on an individual and its total length.
A generalized linear model was fitted, using the number of encounters as the response variable and search effort (in hours) and year as explanatory variables, to establish whether encounter rate changed over the course of the study. As the dataset ended in June 2010, a second model was fitted to 6-month periods, using the number of encounters as the response variable and search effort and 6-month block as explanatory variables. A quasi-poisson error structure was used for both analyses because of overdispersion of residual deviance. A third model was used to test for a change in encounter duration during the study period, using log-transformed encounter duration as the response variable and month and year as explanatory variables. We used χ2 to test for a population-level increase in avoidance response over the five 6-month periods. A generalized linear mixed effects model was used to test whether the likelihood of avoidance was related to the number of previous encounters in which the whale shark had been successfully identified and its behaviours recorded over the study period. We used the Wildbook library to establish how many previous encounters had been recorded for each individual shark. A binomial score for avoidance was used as the response variable, encounter number as the explanatory variable, and shark identity as a random factor. Significance was accepted at the 95% confidence interval in all analyses.
Results
We recorded a total of 689 whale shark encounters from 328 trips during January 2008–June 2010, with a total search effort of 476.5 hours. The mean encounter rate was 2.73 ± SD 0.17 per trip, with 0–14 encounters recorded on each trip, equating to 1.46 ± SD 0.10 sharks per hour of search effort. At least one shark was sighted on 77.1% of trips. There was no significant trend in sightings over the course of the study (linear regression, df = 27, P = 0.260; Fig. 2). A total of 142 sharks were positively identified using the Wildbook photo-identification library. Of 128 sexed sharks, 33 were female (25.8%) and 95 were male (74.2%), which was significantly different from a 1 : 1 sex ratio (χ2 1 = 30.031, P ≤ 0.001). The mean total length was 5.85 ± SD 1.28 m (range 3–8.5 m) for females and 6.14 ± SD 1.24 m (range 3–9.5 m) for males (Fig. 3), with no significant difference between the sexes (t-test, t = −1.144, df = 125, P = 0.255). The total number of encounters with identified sharks (counted as the number of days on which interactions with a particular shark were recorded) was 1–5 over the course of the study, although the inclusion of additional data from the Wildbook library showed that 42% of the individual sharks considered in this study had been exposed to swimmers prior to the start of this work in 2008. Overall, sharks were encountered on a mean of 3.08 ± SD 2.13 occasions, with up to 12 encounters per individual (Fig. 4). The reliance on suitable photographs having been submitted to the library means that these figures represent the minimum number of previous encounters. Scarring was observed on 53.3% of identified individuals and whale sharks were observed feeding during 19.5% of all encounters.

Fig. 2 Mean (± SD) number of whale sharks Rhincodon typus sighted per daily trip during the 30-month sampling period. Numbers above the bars denote the number of sampling days in that month.

Fig. 3 Frequency distribution of the estimated lengths of photo-identified sharks.
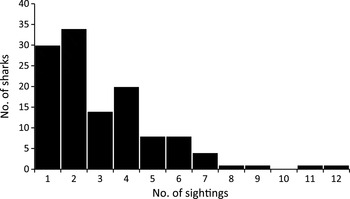
Fig. 4 The total number of sightings (unique encounter days) for each photo-identified shark.
The mean duration of encounter was 9 minutes 46 s ± SD 8 minutes 18 s (n = 613). One or more avoidance responses were recorded during 67.5% of encounters. Evaluation of 184 encounters with 132 identified sharks indicated that encounter duration was significantly related to expressed avoidance response (generalized linear mixed model, χ2 3 = 15.046, P = 0.002). The mean duration of encounter when a shark showed no avoidance was 12 minutes 37 s ± SD 8 minutes 58s (n = 190), which is significantly longer than encounters with sharks that exhibited avoidance (generalized linear mixed model, χ2 1 = 14.255, P < 0.001). There was no significant difference in the duration of encounters where one or more avoidance behaviours were expressed (generalized linear mixed model, χ2 2 = 0.791, P = 0.673); mean duration of such encounters was 8 minutes 25 s ± SD 7 minutes 21 s (n = 397). A binomial response (avoidance or no avoidance) was therefore used in subsequent analyses.
All potential explanatory variables were present in 170 encounters and, after controlling for multiple encounters with individual sharks, 120 encounters were available to test for other significant variables affecting encounter duration. A linear mixed effects model, with year and month retained as nested random effects, demonstrated that scarring was highly significant (linear mixed effects, χ2 2 = 10.953, P = 0.004). Encounters with individuals that had no apparent scarring were significantly shorter than with individuals that had some degree of scarring (linear mixed effects, χ2 1 = 7.927, P = 0.005); the severity of the scarring did not significantly affect encounter duration (linear mixed effects, χ2 1 = 3.027, P = 0.082). There was no significant relationship between the total length of an individual and the degree of scarring observed (ANOVA, F (2,627) = 2.390, P = 0.122). Encounters were significantly longer when sharks were feeding (linear mixed effects, χ2 1 = 5.608, P =0.018) and when more swimmers were present (linear mixed effects, χ2 1 = 5.580, P = 0.018).
The number of whale sharks encountered was not correlated with search effort between years (ANOVA, F (1,249) = 1.134, P = 0.288). There was a significant difference in encounter rates between years (ANOVA, F (2,249) = 5.446, P = 0.005) but analysis of 6-month blocks indicates that the source of this difference was the second half of 2008, for which July and August were not included (ANOVA, F (4,247) = 8.591, P ≤ 0.001). Encounter rate was generally low during the austral winter, so excluding these 2 months may have affected this result. No other variables were significant. There was no significant difference in the frequency of avoidance behaviours expressed between the five 6-month periods (χ2 4 = 4.34, P = 0.362). Encounter duration was significantly shorter in 2008 (8 minutes 2 s ± SD 8 minutes 32 s) than in 2009 (10 minutes 38 s ± SD 8 minutes 7 s) and 2010 (10 minutes 37 s ± SD 7 minutes 56 s; ANOVA, F (2,610) = 17.925, P ≤ 0.001). A total of 204 encounters with 142 whale sharks were analysed to establish that the number of previous encounters with swimmers did not affect the likelihood that a whale shark would display avoidance behaviour (generalized linear mixed model, χ2 1 = 1.693, P = 0.193).
Discussion
We found no significant longitudinal effects on encounter duration or the raw proportion of avoidance behaviours expressed over the 30-month study period. Furthermore, there was no significant relationship between the number of previous tourist encounters and the likelihood of avoidance behaviours being displayed by individual sharks. At this stage there is no evidence of short-term avoidance behaviours translating into medium- to longer-term behavioural change among the sharks present in Mozambican waters.
The potential negative effects of tourism may be ameliorated by the ecology and population structure of whale sharks in Mozambique. The majority of sharks encountered were male (74%). Maturity in male sharks occurs when they reach a total length of c. 8 m, based on records from Ningaloo Reef (Norman & Stevens, Reference Norman and Stevens2007), and in female sharks at a total length of > 8.7 m, based on South African and other records (Beckley et al., Reference Beckley, Cliff, Smale and Compagno1997; Norman & Stevens, Reference Norman and Stevens2007). Given that no sharks of total length > 9.5 m or < 3 m were encountered, most sharks were immature and were probably several years old at the time of first sighting (Wintner, Reference Wintner2000). Therefore, tourism at this site is unlikely to interfere with reproduction, if it does in fact occur in these waters. The majority (52%) of sharks had only one or two encounters recorded on the Wildbook photo-identification library by 30 June 2010 (with the earliest record from 2003), including from this study, suggesting that many of the sharks are transient to the area, as noted in other aggregations (Holmberg et al., Reference Holmberg, Norman and Arzoumanian2008; Rowat et al., Reference Rowat, Speed, Meekan, Gore and Bradshaw2009; Fox et al., Reference Fox, Foisy, de la Parra Venegas, Galván Pastoriza, Graham and Hoffmayer2013). There was no evidence of a learnt avoidance behaviour amongst individual sharks and, based on data from the Philippines (Quiros, Reference Quiros2007), a degree of habituation may be more likely to occur.
The percentage of whale sharks observed feeding during daylight hours at Tofo (19.5%) is comparable to the rates reported at Donsol in the Philippines (13% in 2004 and 36% in 2005; Quiros, Reference Quiros2007) but substantially lower than the 69% reported from Bahía de Los Angeles, Mexico, where researchers targeted feeding whale sharks (Nelson & Eckert, Reference Nelson and Eckert2007). Contrary to results from the Philippines, where feeding sharks were 1.84 times more likely to exhibit a dive response in the presence of swimmers (Quiros, Reference Quiros2007), encounter durations in this study were significantly longer when feeding behaviour was observed. Whale sharks are flexible in their foraging strategies, depending on prey type and vertical distribution (Graham et al., Reference Graham, Roberts and Smart2006; Nelson & Eckert, Reference Nelson and Eckert2007; Taylor, Reference Taylor2007; Brunnschweiler et al., Reference Brunnschweiler, Baensch, Pierce and Sims2009; Motta et al., Reference Motta, Maslanka, Hueter, Davis, de la Parra and Mulvany2010), so the effects of tourism on feeding are likely to be context-specific.
Whale shark encounters were spatially aggregated along 9 km of coast immediately south of Tofo Beach. Although the sharks are also sighted more widely along the Mozambican coast (Cliff et al., Reference Cliff, Anderson-Reade, Aitken, Charter and Peddemors2007), the small size of the primary aggregation area and its almost complete daily search coverage by commercial operators suggest the possibility of displacement if whale sharks have negative interactions with swimmers or boats. Over half (53%) of identified sharks had some form of scarring, although not all of these were from anthropogenic sources such as propeller strikes. This percentage is lower than that recorded in Djibouti, where propeller or boat strike scars were observed on 65% of identified sharks (Rowat et al., Reference Rohner, Pierce, Marshall, Weeks, Bennett and Richardson2007), but emphasizes the importance of working with skippers to ensure that a safe minimum distance from the sharks is maintained (Pierce et al., Reference Pierce, Mendez-Jimenez, Collins, Rosero-Caicedo and Monadjem2010). Although tourism operators have not perceived a shift in whale shark distribution, the lack of standardized spatial sampling coverage precludes a definitive assessment.
Our results support the use of encounter duration as a means of measuring the effect of swimmer presence on whale shark behaviour, as sharks that are disturbed will routinely end an encounter by diving or increasing their swimming speed. However, it is important to note that encounter duration, although a useful proxy of avoidance, may only be able to provide resolution at the binomial scale (avoidance or no avoidance). Encounter duration has previously been used to quantify avoidance by Hector's dolphins Cephalorhynchus hectori of swimmers in New Zealand (Bejder et al., Reference Bejder, Dawson and Harraway1999) and to assess long-term trends in encounters with dwarf minke whales Balaenoptera acutorostrata on the Great Barrier Reef, Australia (Birtles et al., Reference Birtles, Arnold and Dunstan2002). However, these studies used different definitions of encounter duration: Bejder et al. (Reference Bejder, Dawson and Harraway1999) defined it as the length of time that a swimmer spent within 200 m of the nearest dolphin and Birtles et al. (Reference Birtles, Arnold and Dunstan2002) defined it as the time between the first sighting of a whale and the end of the vessel's contact with that whale. Here we define encounter duration as the time between the first swimmer entering the water in the presence of a whale shark and the last swimmer returning to the boat. This definition could be less useful in areas with significant currents or where the boat would take longer to retrieve swimmers.
We found a positive relationship between the number of swimmers and encounter duration. This result may be an artefact of the definition in use, as larger groups of swimmers are likely to contain members with a wider range of swimming abilities. The presence of more able swimmers is likely to lead to an increase in encounter duration, as they are able to maintain visual contact with a shark for longer than slower or less fit individuals. Another important point to consider in future work is the number of swimmers that are actually in contact with the shark from a behavioural perspective, investigating how swimmer proximity may influence these results. Future studies that rely on in-water observers could trial re-defining encounter duration as the time between the observer's first and last visual contact of the shark underwater, to reduce the influence of swimming ability and group size on results.
Consistent with results from Quiros (Reference Quiros2007) but contrary to Norman (Reference Norman1999) encounter duration was significantly longer with scarred whale sharks. This could be because scarred individuals have slower reaction times and a reduced level of agility as a consequence of their previous injuries. However, the severity of scarring did not significantly alter encounter duration. Another hypothesis is that older individuals, who would presumably be more likely to have accumulated scars, show less avoidance than younger individuals. However, we found no significant relationship between shark size and the severity of scarring. A third plausible explanation is that some sharks have a slower natural reaction time to potential threats and are therefore more likely to accumulate injuries than sharks with faster reactions. Sharks may also vary in their inquisitiveness. Further research is required to confirm or refute these hypotheses.
A mean of 11.4 swimmers per encounter was recorded. This exceeds the maximum recommended by most management plans, which limit swimmer numbers to three in Mexico (Remolina Suárez et al., Reference Remolina Suárez, Pérez Ramírez, González Cano, de la Parra Venagas, Betancourt Sabatini, Trigo Mendoza, Irvine and Keesing2007) and 10 in Australia (Catlin & Jones, Reference Catlin and Jones2010), although these limits may be partially influenced by operational constraints in those locations. From a management perspective it is also important to consider customer satisfaction with the experience, which can be significantly reduced in crowded scenarios (Davis et al., Reference Davis, Banks, Birtles, Valentine and Cuthill1997).
The inclusion of well-defined and easily observed parameters of known significance that can be recorded by either trained specialists (e.g. researchers) or non-specialist observers (e.g. tour operators or volunteers) would increase the amount of information available, enabling routine sustainability assessments and facilitating inter-site comparisons (Graham, Reference Graham2007). We have created a template data collection sheet (Supplementary Material 1), which is currently in use in Mozambique. We also encourage the submission of standardized photographs of all whale sharks encountered to the global whale shark photo-identification library (Wild Me, 2003). This facilitates the incorporation of individual-based analyses into future studies, concurrently enabling more accurate regional and ocean-wide studies of population size and interconnectivity (Graham, Reference Graham2007; Brooks et al., Reference Brooks, Rowat, Pierce, Jouannet and Vely2010).
With increasing global interest in human interactions with whale sharks there is a clear need for the implementation of monitoring initiatives at tourism sites to understand and avoid long-term anthropogenic effects. Other important whale shark tourism sites, such as those in Mexico, the Seychelles and Western Australia, have similar characteristics in terms of population structure. The results of this study demonstrate that encounter duration is a useful metric for assessing whale shark behaviour, and provide a template for future studies in Mozambique and other locations.
Acknowledgements
We thank the All Out Africa volunteers and staff for their efforts in collecting the majority of the data used in this study, and Marine Megafauna Foundation staff, volunteers and students who also contributed. We thank the developers of, and contributors to, the Wildbook whale shark database. The support of Casa Barry Lodge and Tofo Scuba throughout the field portion of the project is greatly appreciated. This study was made possible by support from the National Marine Aquarium (UK), Shark Foundation, GLC Charitable Trust, Project AWARE Foundation, the Rufford Small Grant Foundation, PADI Foundation, Ocean Revolution, Fondation Ensemble and private donors. We thank Philip Dearden, Adrian Gutteridge, Conrad Speed and the anonymous reviewers for their comments on this article.
Biographical sketches
Peter Haskell specializes in behavioural ecology and is interested in the role that tourism can play in elasmobranch conservation. Andrew McGowan's research interests include avian cooperative breeding, conservation of sea turtles, and tropical seabird populations. Anna Westling is a coral reef ecologist interested in citizen science. Adriana Méndez-Jiménez is interested in the human dimension of the environment, and in the way people perceive environmental issues. Christoph Rohner is interested in the movement and feeding ecology of large pelagic animals, and linking their behaviour to biological and oceanographic drivers. Kym Collins specializes in the behaviour and acoustics of marine mammals. Marcela Rosero-Caicedo is interested in marine conservation and specializes in scientific and environmental communication in volunteer programmes and local communities. Jodi Salmond studies Australian reef ecosystems in a global context, specializing in citizen science and marine education. Ara Monadjem is interested in the application of quantitative ecological techniques to conservation-related problems in small mammals and birds. Andrea Marshall studies the biology, ecology, monitoring and management of manta rays globally. Simon Pierce is a marine conservation ecologist specializing in whale sharks.





