Introduction
Although Ernst Schwarz described the dryas monkey Cercopithecus dryas in 1932, it remains one of Africa's most enigmatic primates. Poor documentation of the type locality, description of Cercopithecus salongo as a new species, and lack of specimens have led to taxonomic confusion (Schwarz, Reference Schwarz1932; Dandelot, Reference Dandelot, Meester and Setzer1971; Thys van den Audenaerde, Reference Thys van den Audenaerde1977; Kuroda et al., Reference Kuroda, Kano and Muhindo1985; Groves, Reference Groves2001). Cercopithecus salongo specimens were eventually determined to be the adult of the juvenile C. dryas type specimen (Colyn et al., Reference Colyn, Gautier-Hion and Thys van den Audenaerde1991), although this remains disputed (Sarmiento, Reference Sarmiento2000; Kingdon, Reference Kingdon2015). Recent genomic studies have confirmed that the dryas monkey is a sister lineage to savannah monkeys of the genus Chlorocebus, suggesting the Cercopithecus group is paraphyletic (Guschanski et al., Reference Guschanski, Krause, Sawyer, Valente, Bailey and Finstermeier2013; van der Valk et al., Reference van der Valk, Gonda, Silegowa, Almanza, Sifuentes-Romero and Hart2020). Here we accept Chlorocebus as the appropriate generic affiliation, pending further analyses of available specimens, and accept salongo as a junior synonym of dryas (Gilbert et al., Reference Gilbert, Gilissen, Arenson, Patel, Nakatsukasa and Hartin press). The dryas monkey's status as the only representative of the Chlorocebus clade found in lowland rainforest highlights its high conservation value as an evolutionarily significant lineage.
The dryas monkey was formerly thought to be a localized relict species, known only from a single population in the Luo Scientific Reserve, Iyondji Community Bonobo Reserve, Kokolopori Bonobo Reserve and forest near Djombolanda Village in Tshuapa Province, Democratic Republic of the Congo (Fig. 1; Kuroda et al., Reference Kuroda, Kano and Muhindo1985; Lokasola, Reference Lokasola2008; Sakamaki et al., Reference Sakamaki, Maloueki, Bakaa, Bongoli, Kasalevo, Terada and Furuichi2016; A. Lokasola, pers. comm., 2016). This small range justified the categorization of the species as Critically Endangered (Hart et al., Reference Hart, Butynski and Hurley2008). However, new information about the dryas monkey's range was reported in 2014, when field assistants from the TL2 Project (named after the landscape between the rivers Tshuapa, Lomami and Lualaba) documented two dryas monkeys killed by hunters in Bafundo Forest, Maniema Province, 400 km south-east of the species’ known range (Hart, Reference Hart2014). This range expansion justified a recategorization of the dryas monkey as Endangered, and emphasized the need for field studies (Hart et al., Reference Hart, Detwiler, Alempijevic, Lokasola and Rylands2019).
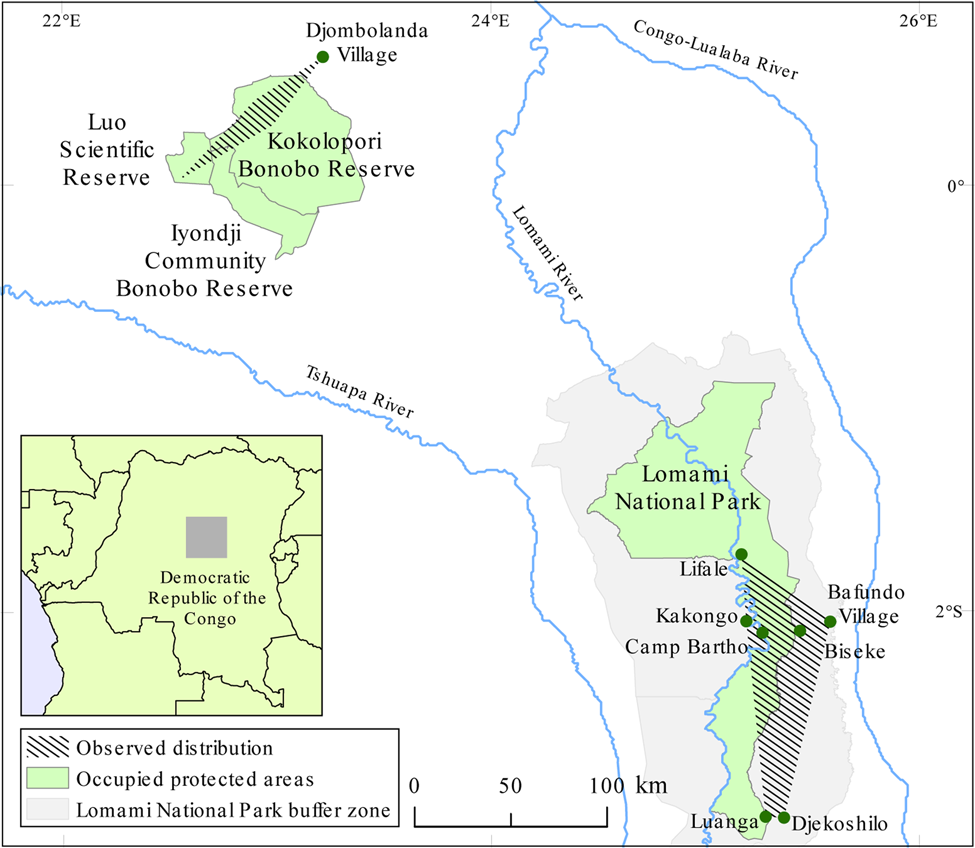
Fig. 1 The global distribution of the dryas monkey Chlorocebus dryas, endemic to the Democratic Republic of the Congo, indicating the location of the Luo–Djombolanda population (Luo Scientific Reserve, Iyondji Community Bonobo Reserve, Kokolopori Bonobo Reserve, Djombolanda Forest) and the Lomami National Park and buffer zone population. Specific locations where dryas monkeys have been observed in Lomami National Park and its buffer zone are labeled.
A limiting factor for investigating the distribution of the dryas monkey is that it is difficult to detect. The TL2 Project did not detect the dryas monkey during the first 7 years of biodiversity surveys, despite documenting 10 other primate species (Hart et al., Reference Hart, Detwiler, Gilbert, Burrell, Fuller and Emetshu2012). Dryas monkeys are primarily reported in secondary forest thickets (Kuroda et al., Reference Kuroda, Kano and Muhindo1985; Lokasola, Reference Lokasola2014). Kuroda et al. (Reference Kuroda, Kano and Muhindo1985) encountered dryas monkeys in the understorey and feeding on the ground, but surveys using camera traps placed at ground level in Iyondji Community Bonobo Reserve and Lomami National Park and its buffer zone failed to detect them (Sakamaki et al., Reference Sakamaki, Maloueki, Bakaa, Bongoli, Kasalevo, Terada and Furuichi2016; J.A. Hart et al., unpubl. data). Although camera traps are widely used to detect and survey cryptic species, it is necessary to develop specific camera-trap placements that could reliably detect dryas monkeys.
Local knowledge has been shown to provide useful information on understudied and cryptic species (Cano & Tellería, Reference Cano and Tellería2013; Nguyen et al., Reference Nguyen, Tran, Hoang, Nguyen, Nguyen and Tran2019). We therefore solicited information on the dryas monkey from residents of villages in the Lomami National Park's buffer zone to determine the distribution of the dryas monkeys and select sites for camera-trap surveillance. We then used camera traps to document stratum and habitat preference and estimate the relative abundance of the dryas monkey at two sites, one each in Lomami National Park and its buffer zone. Specifically, we tested the following hypotheses: dryas monkeys (1) prefer the understorey–mid canopy strata, (2) prefer disturbed patches over mature forest, and (3) would be detected more frequently in Lomami National Park than in its buffer zone, where hunting is more common. We also developed a species-specific camera-trap method to detect the species.
Study area
This study involved two field surveys: Phase I (October 2016–July 2017) and Phase II (August 2017–March 2018) at two sites in Lomami National Park and its buffer zone in Maniema Province, Democratic Republic of the Congo. Lomami National Park encompasses 8,874 km2 of lowland rainforest and savannah bisected by the Lomami River. The Park was established in 2016 and with its buffer zone covers c. 29,000 km2 in the TL2 Landscape. The landscape has < 150 villages, the residents of which practice shifting agriculture, fishing and hunting in the Park's buffer zone.
We selected Bafundo Forest in the buffer zone for camera-trap surveillance, starting at the location where the first reported dryas monkey was killed by a hunter. This forest was the site of Bafundo Village during 1935–1956, before its residents moved to their current location 3 km to the south-west. We established the second survey site 40 km west of Bafundo Village, at Camp Bartho in Lomami National Park after a ranger patrol team saw a dryas monkey there in 2014. Camp Bartho was occupied by one family during 2006–2013 and included five dwellings and several gardens. Since then, the family moved their camp to the west bank of the Lomami River, outside the Park.
Methods
Distribution
We created a dryas monkey illustration matching the pelage of the adult male specimen killed in Bafundo Forest (Fig. 2). We used it to create a poster that invited people to come forward with information about sightings of the species, locally known as inoko. We conducted informal interviews opportunistically when an informant volunteered information. We distributed the poster in villages and incorporated it into the species identification material used by patrol teams. We trained the teams in species identification and equipped them with cameras to take photographs of any dryas monkeys seen during patrols. If a patrol team encountered a dryas monkey or if one killed by a hunter was photographed, we considered these as confirmed occurrences (Hart, Reference Hart2019).

Fig. 2 Illustration of an adult male dryas monkey Chlorocebus dryas, drawn from photographs of an individual killed by a hunter in the Bafundo Forest, for use in educational material distributed in local communities, to help bio-monitoring patrol teams inform people of the protected status of the inoko, and to solicit information on the occurrence of the species.
Camera-trap survey design
We developed a multi-strata camera-trap technique with a placement of three camera traps at each survey point, one each monitoring the ground, understorey and canopy. We positioned the camera traps on game paths (0.2–0.5 m above ground), shrubs and liana tangles (1.5–10.0 m), and horizontal limbs (14.9–29.0 m) forming pathways to adjacent tree crowns (Gregory et al., Reference Gregory, Carrasco Rueda, Deichmann, Kolowski and Alonso2014). We used the single-rope technique to access the canopy, and the double-rope technique to move to the desired camera-trap location when needed (Maher, Reference Maher2010).
We used 55 Bushnell (Overland Park, USA) camera traps (models Trophy Cam 119636, Trophy Cam 119736, Trophy Cam Aggressor 119774, Essential E3 119837). We programmed them to take 60-second high-quality videos over a 24-hour period using high sensitivity and a 1-second rest period.
Phase I was a strategic survey to increase our chances of detecting dryas monkeys, using 20 camera-trap placements in Bafundo Forest and Camp Bartho (Fig. 3). We set placements in locations where informants had seen dryas monkeys, or in areas with a dense understorey, where informants had indicated dryas monkeys prefer to forage. We set two additional camera traps in the understorey at the edge of a recently abandoned garden at Camp Bartho.

Fig. 3 The spatial relationship between sampling points in the strategic and systematic surveys, and the distribution of dryas monkey detections at the Camp Bartho and Bafundo Forest survey sites.
Phase II was a systematic survey, to eliminate bias in detection rates, in Bafundo Forest and Camp Bartho. We selected 24 sampling points 500 m apart in 1.5 km2-grid cells (Fig. 3). The mean distance of each camera-trap placement to the pre-determined sampling point was 23.8 m ± SD 13.2 m.
Video processing and analysis
We grouped videos into an event if ≤ 30 minutes passed between dryas monkey videos from the same camera trap. We grouped trap-days per stratum to compare trap effort between strata. Trap-days are the total number of days a camera trap was functional in the field. If a camera trap was not functioning when we retrieved it, we considered the date and time of the last video recorded as the last trap-day.
We calculated the dryas monkey detection rate per stratum (dryas monkey events/camera-trap days × 100) to compare events across surveys with different trap effort. To determine if dryas monkeys show preference for a particular stratum, we compared detections per stratum between sites and surveys using the Kruskal–Wallis rank sum test. To calculate the minimum trap effort required to detect a dryas monkey from our data, we determined the probability distribution of time between events by fitting the observed detection rates to a Poisson point process for each survey (Supplementary Fig. 1).
Habitat modeling
To create a predictive model of dryas monkey habitat preference, we considered six a priori covariates. As both local knowledge and previous studies indicated dryas monkeys prefer disturbed sites (Kuroda et al., Reference Kuroda, Kano and Muhindo1985; Lokasola, Reference Lokasola2014), we recorded covariates related to forest structure in a 20-m diameter plot at all 24 sampling points in Phase II. We recorded per cent canopy cover, canopy height, and understorey complexity (the number of times vegetation touched a 5-m vertical pole; modified from Suarez-Rubio et al., Reference Suarez-Rubio, Ille and Bruckner2018) at 5 and 10 m height in the 4 cardinal directions from the sampling point, recorded the number of trees and their diameter at breast height (DBH; > 10 cm), and noted the presence or absence of fallow fields. We examined any potential differences in habitat characteristics between Bafundo Forest and Camp Bartho using a multivariate analysis of variance.
We used logistic regression to model the presence/absence of dryas monkeys at camera-trap sampling points as a function of habitat covariates. We did not record DBH, number of trees, or understorey complexity during Phase I, and therefore we used the mean values from Phase II for these variables. To avoid problems associated with collinearity between covariates, we used variable inflation factors calculated with the ISLR 2.1 package in R 3.6.1 (R Core Team, 2019). Covariates with a variable inflation factor > 10 were excluded from the model. We determined the covariate(s) that had the most predictive power from the global model, from which we selected the covariate(s) with the lowest Akaike's information criterion (AIC) score and P ≤ 0.05. We analysed variation between the null and residual deviance using analysis of variance. To evaluate candidate models, we calculated AIC corrected for small sample size (AICc) and maximum likelihood values using the MuMin 1.43.6 package, to determine the fit of the global model and select the most parsimonious model. We examined the relative importance of each variable using the per-variable sum of model weights. We determined variable importance based on the predictive power of each variable in the model, and then calculated the probability of detecting dryas monkeys for the important variables.
Results
We confirmed seven occurrences of dryas monkeys in Lomami National Park and its buffer zone during 2014–2019 based on opportunistic reports provided by local residents and park patrols (Table 1). Observations were confirmed in the edge of gallery forest, at sites of current or historical anthropogenic activity, and in an area of natural liana abundance in mature forest (Supplementary Fig. 2). The dryas monkey's range (its extent of occurrence) in Lomami National Park and its buffer zone is 3,453 km2 (Fig. 1).
Table 1 Data on the occurrence of the dryas monkey Chlorocebus dryas collected opportunistically during patrols in Lomami National Park and its buffer zone, Democratic Republic of the Congo (Fig. 1).

Camera traps detected dryas monkeys at both sites and in both surveys (Plate 1). Detection frequencies were higher in Bafundo Forest than in Camp Bartho. The minimum number of days of accumulated trap effort required for a 95% certainty of detecting dryas monkeys was 135–365 (Table 2).

Plate 1 Still-frame from a camera-trap video of an adult male dryas monkey Chlorocebus dryas in Bafundo Forest in the buffer zone of Lomami National Park (Fig. 1).
Table 2 Frequency of detection of dryas monkeys in the forest understorey (1.5–10 m) and the estimated minimum trap effort required for a 95% probability of detecting the species, using camera traps in the Phase I and Phase II surveys (see text for details and Fig. 1) in Bafundo Forest and Camp Bartho.

1 Number of events per 100 trap days.
2 Trap effort (camera-trap days) required to be 95% certain a dryas monkey will be detected if present at a site.
3 Actual trap effort accumulated before detecting a dryas monkey in the survey.
Camera traps accumulated 1,742 trap-days on the ground, 2,821 trap-days in the understorey and 2,927 trap-days in the canopy. Understorey camera traps recorded 32 dryas monkey events, canopy camera traps detected a single event, and dryas monkeys were not detected by camera traps at ground level. We found no significant difference in detection height across surveys (P = 0.996; Fig. 4).

Fig. 4 The height above ground at which dryas monkeys were detected by camera traps in strategic (I) and systematic (II) surveys in the Bafundo Forest and Camp Bartho. The boxes represent the median height with upper and lower quartiles where dryas monkeys were detected (25% greater and 25% lesser than the median); whiskers represent maximum/minimum values, and circles outliers.
Some aspects of forest structure differed significantly between Bafundo Forest and Camp Bartho (Table 3). There were more fallow fields and the forest understorey was more complex in Bafundo Forest (Plates 2 & 3), and tree density was significantly higher in Camp Bartho.
Table 3 Comparison of six habitat characteristics of Bafundo Forest and Camp Bartho, with the mean and standard deviation of each covariate per site, and the F and P values from the multivariate analysis of variance.

*P < 0.05; **P < 0.01.
1 Number of times vegetation touched a vertical pole 5 m in height.

Plate 2 Understorey structure typical of fallow fields in Bafundo Forest (left) and mature forest at Camp Bartho (right) in the buffer zone and Lomami National Park, respectively.
All six covariates had variance inflation factors < 10, and therefore we used all covariates in the logistic model. In the global model, fallow fields was the only covariate identified as a significant predictor of dryas monkey presence (Table 4). Importance tests of the covariates in the best-fit models (Table 5; see Supplementary Material 1 for all models) indicate fallow fields was the covariate of greatest importance (0.99). The likelihood of detecting dryas monkeys on the edge of fallow fields was higher (0.67) than in mature forest (0.2).
Table 4 The six covariates tested for inclusion in multivariate logistic models of habitat use by dryas monkeys at two sites in Lomami National Park and its buffer zone, selected based on literature and local ecological knowledge, with the mean and standard deviation (x̅ ± SD) and probability of each covariate in the model. We collected covariate data strategically from disturbed forest where local hunters suggested dryas monkeys were likely to be encountered (n = 21) and systematically sampled every 500 m at the same sites (n = 22).

* P < 0.05.
Table 5 Habitat logistic regression models for habitat use by dryas monkeys in Camp Bartho and Bafundo Forest.

1 AICc, Akaike's information criterion adjusted for small sample size.
2 ΔAICc, difference in Akaike's information criterion adjusted for small sample size in relation to the top model.
3 AICcWt, relative likelihood of the model adjusted for small sample size.
Discussion
Our study of the dryas monkey population in Lomami National Park and its buffer zone adds to our knowledge of the distribution, stratum preference, and habitat use of this enigmatic species. Most of the new records of the species came from the knowledge of local people. People that directly depend on forest resources are often knowledgeable about local biodiversity (Gadgil et al., Reference Gadgil, Berkes and Folke2019). Even rapid, unstructured interviews can be useful, but reports need to be confirmed, as we did, with camera traps or other complementary methods (Nguyen et al., Reference Nguyen, Tran, Hoang, Nguyen, Nguyen and Tran2019).
The known distribution of dryas monkeys includes both banks of the Lomami River, a major tributary of the Congo River (Fig. 4). Therefore, the Congo River may be the only fluvial barrier to the species' distribution. No population has been confirmed west of the Tshuapa River, despite assumptions that the species occurs further to the west in Salonga National Park (Thys van den Audenaerde, Reference Thys van den Audenaerde1977). Kingdon's (Reference Kingdon2015) suggestion that dryas monkeys could occur at more westerly localities south of the Congo River needs to be investigated with further surveys.
Our camera-trap placements detected dryas monkeys almost exclusively in the understorey across all surveys, regardless of canopy structure, supporting our hypothesis that the species prefers the understorey–mid canopy strata. A few monkeys were recorded descending out of view towards the ground. Dryas monkeys have been seen foraging on Aframomum and Megaphrynium (Kuroda et al., Reference Kuroda, Kano and Muhindo1985; Kingdon, Reference Kingdon2015), both abundant herbs in fallow fields in the Bafundo Forest (Plate 3). However, as the camera traps on the ground did not detect dryas monkeys, it seems unlikely they travel on the ground. Only one camera trap in the canopy detected a dryas monkey, suggesting they rarely travel in the upper canopy.

Plate 3 View from the canopy of a fallow field in Bafundo Forest, characterized by sparse trees, dense herbs, and lianas.
Dryas monkeys showed a preference for the edges of fallow fields, supporting our hypothesis that the species prefers disturbed patches over mature forest. Dryas monkeys were detected most frequently in Bafundo Forest during the Phase II survey, where we encountered the greatest area of anthropogenic disturbance. The small (2.2–3.3 kg) body size of dryas monkeys may be adapted to living in such dense vegetation (Kuroda et al., Reference Kuroda, Kano and Muhindo1985).
Some primates prefer disturbed microhabitats in otherwise mature forest (Bourlière, Reference Bourlière1985). Two West African strepsirhines, Arctocebus calabarensis and Euoticus elegantulus, are restricted to liana-rich microhabitats (Charles-Dominique, Reference Charles-Dominique1977). In Ituri Forest (1.6711° N, 28.4874° E), Cercopithecus ascanius specializes in foraging for arthropods in forest gaps (Thomas, Reference Thomas1991). In Lomami National Park and its buffer zone dryas monkeys use mature forest, but prefer disturbed forest patches.
Despite being hunted in the buffer zone, dryas monkeys were mostly detected in Bafundo Forest, and thus our hypothesis that the species would be detected more in Lomami National Park than in its buffer zone was not supported by our data. It was not possible from our data to separate the effects of hunting and fallow fields, but the gains received by exploiting disturbed sites must presumably outweigh the cost of being hunted. We are not aware of any reports of dryas monkeys in local or regional bushmeat markets, and thus the species may be harvested at a lower rate than other monkey species. Local people in Bafundo Village indicated the monkey is too small to send to market and the species’ preference for dense thicket makes them difficult to shoot.
Although hunting and habitat loss are causing global declines in primate populations (Estrada et al., Reference Estrada, Garber, Rylands, Roos, Fernandez-duque and DiFiore2017), African primates have adapted to forest edge conditions in the past during dry climatic periods (De Almeida-Rocha et al., Reference De Almeida-Rocha, Peres and Oliveira2017). Dryas monkeys may be exploiting disturbed patches in the Congo Basin, as Chlorocebus does along the Basin's periphery. The sightings in Djekoshilo and Luanga at the southern limit of the rainforest block are within 75 km of the northern range limit of Malbrouck's monkey Chlorocebus cynosuros (Sarmiento, Reference Sarmiento, Butynski, Kingdon and Kalina2013). The dryas monkey's use of gallery forest, and the southern extent of its range, should be explored further to determine possible sympatry between dryas and Malbrouck's monkeys.
We were able to detect the dryas monkey with our placements of three camera traps arranged vertically in different forest strata, and at least 365 trap-days are required to achieve a 95% detection probability, although dryas monkeys may be detected sooner in suitable habitat. The cameras that detected the species most frequently were those in the lower strata of the canopy and understorey, at 2–10 m above ground. Because foliage was dense and branches thin at 10–15 m above ground, this stratum was not suitable for placement of camera traps, although we observed dryas monkeys at this height (Supplementary Fig. 3).
Our findings indicate that Lomami National Park and its buffer zone contain the greatest extent of the species’ known range (Fig. 1), and Lomami is the only National Park with a confirmed population. High heterozygosity and low inbreeding measures from genomic analysis of a hunter-killed dryas monkey (collected on 26 October 2014) from Bafundo Forest suggest that it was a representative of a larger contiguous population (van der Valk et al., Reference van der Valk, Gonda, Silegowa, Almanza, Sifuentes-Romero and Hart2020). We recommend that researchers and conservationists working in the central basin of the Democratic Republic of the Congo mobilize local knowledge to identify other areas where the dryas monkey occurs and confirm any reports using our camera trap placement technique.
Acknowledgements
We dedicate this manuscript to Pablo Ayali (1981–2018) for his commitment to the dryas monkey project and the conservation of Lomami National Park. This research was funded by the Lukuru Foundation, Margot Marsh Biodiversity Foundation (001378), Mohammed Bin Zayed Species Conservation Fund (162513850), Florida Atlantic University, Primate Conservation Incorporated (1322), International Primatological Society, Dynamic Youth Community, and crowd funding. We thank Erik Noonburg and Michael Lawes for statistical support and helpful comments; Jeanne Pierre Kapale, Henri Silegowa, Aimedo Onale, Boniface Kamanya, Chief Onombe Djecko and other informants who reported dryas monkey observations; Marten Balimu, Reddi Bosisa and John I. Mponga for assistance with camera trapping; and Martin Fisher, Jef Dupain and an anonymous reviewer for their suggestions.
Author contributions
Study design: DA, KD, JH, TH; fieldwork: DA; data analysis: DA; writing : DA, KD, JH, TH.
Conflicts of interest
None.
Ethical standards
This non-invasive research used no-flash camera traps. L'Institut Congolais pour la Conservation de la Nature granted research permission to work in Lomami National Park. The local chiefs of Bafundo, Likandjo and Bote villages and the sector chief of Maniema Province granted permission to access the forest. Florida Atlantic University's Institutional Review Board (916126-4) and the Institutional Animal Care and Use Committee (A16-27) approved our research protocol.













