Introduction
Delirium is an acute neuropsychiatric disorder in medically ill patients, characterized by disturbances in consciousness or attention and cognition, caused by different underlying somatic etiologies, typically with abrupt onset and fluctuating course (American Psychiatric Association, 2000). Delirium is a prevalent and distressing complication in patients with advanced cancer (Kang et al., Reference Kang, Shin and Bruera2013). It can be categorized into different motor subtypes — hypoactive, hyperactive, and a mixed type — which may impact prognosis to different degrees (Boettger et al., Reference Boettger, Jenewein and Breitbart2015). The hypoactive is the most prevalent clinical subtype and most frequently manifests in cancer patients (Fang et al., Reference Fang, Chen and Liu2008).
In cancer patients, the reported prevalence of delirium varies according to the stage of disease, treatment modalities used, healthcare setting, and diagnostic tools applied (Bush et al., Reference Bush, Lawlor and Ryan2018). While delirium affects approximately 18% of cancer patients admitted to oncology or internal medicine units (Ljubisavljevic and Kelly, Reference Ljubisavljevic and Kelly2003), delirium occurs in up to 47% of patients with advanced cancer (Fang et al., Reference Fang, Chen and Liu2008) and in 88–93% in the terminally ill (Bush et al., Reference Bush, Tierney and Lawlor2017; Seiler et al., Reference Seiler, Schubert and Hertler2019).
The etiology of delirium in cancer patients is often multifactorial, arising from a combination of risk factors, typically referred to as either “predisposing” or “precipitating” (Inouye et al., Reference Inouye, Westendorp and Saczynski2014). Predisposing factors refer to preexisting characteristics — like male gender, older age, frailty, and hearing, visual, and cognitive impairment — that increase a patient's risk of developing delirium (Seiler et al., Reference Seiler, Schubert and Hertler2019). In contrast, precipitating factors are factors that arise at some point in time and increase the likelihood that delirium will become manifest (Bush et al., Reference Bush, Lawlor and Ryan2018).
Importantly, delirium negatively impacts survival, especially in patients with advanced disease (de la Cruz et al., Reference de la Cruz, Ransing and Yennu2015), and is associated with unfavorable short- and long-term outcomes, including increased morbidity and mortality, prolonged hospital length of stay (LOS), and the need for long-term care institutionalization, resulting in increased healthcare requirements and costs (Boettger et al., Reference Boettger, Jenewein and Breitbart2015; Schubert et al., Reference Schubert, Schurch and Boettger2018). Taking into consideration the negative sequelae of delirium, it is worth noting that delirium is often a preventable, treatable, and reversible condition. However, delirium in cancer patients is frequently unrecognized, misdiagnosed, and therefore, inappropriately treated (Wada et al., Reference Wada, Wada and Wada2010). Identifying delirium risk factors facilitates the early detection of delirium and, thereby, can reduce associated risks and adverse outcomes.
Reviewing the scientific literature reveals that different patient cohorts exhibit different risk factors for delirium (Inouye et al., Reference Inouye, Westendorp and Saczynski2014; Schubert et al., Reference Schubert, Schurch and Boettger2018; Seiler et al., Reference Seiler, Schubert and Hertler2019). A growing body of the literature on risk factors of delirium factors among palliative care patients has been published (Lawlor et al., Reference Lawlor, Gagnon and Mancini2000; Fang et al., Reference Fang, Chen and Liu2008; Seiler et al., Reference Seiler, Schubert and Hertler2019), though comprehensive analyses providing information on risk factors in patients admitted to oncology or hematological units have been limited (Kang et al., Reference Kang, Shin and Bruera2013).
Therefore, this study sought (1) to examine the prevalence of delirium in cancer patients, (2) to explore predisposing and precipitating factors for delirium by comparing delirious and non-delirious patients, (3) to identify the most impactful predisposing and precipitating risk factors, and (4) to compare survival curves in delirious and non-delirious cancer patients.
Methods
Study design, patients, and procedures
This study is part of the Delir-Path, a large prospective observational project that aimed to improve the prevention and facilitate the early detection and management of hospital-acquired delirium in surgical and intensive care patients (Schubert, Reference Schubert2013–2015). Within the Delir-Path project, patients were recruited across 43 departments at the University Hospital Zurich, Switzerland, which is a 900-bed tertiary-care center. A total of 39,432 patients with clinical evidence of incident delirium were enrolled between January 2014 and December 2014 and considered eligible for inclusion. The following exclusion criteria — i.e., (1) age < 18 years; (2) LOS < one day; (3) missing delirium scores [i.e., Delirium Observation Screening (DOS)] — resulted in a total of 29,278 eligible patients [see results published elsewhere (Schubert et al., Reference Schubert, Schurch and Boettger2018)]. Out of these, 1,350 patients were managed at the Department of Medical Oncology and Hematology (Figure 1). For the purpose of this study, only hematological malignancies were included into analyses and classified as hematological diseases; any other hematological diseases such as coagulation disorders were excluded. In addition, patients undergoing hematopoietic stem cell transplantation were not included into our patient cohort as these patients were managed at a different ward. For the purpose of this study, the term “cancer patients” will be used as an umbrella term that refers to patients with oncological and hematological malignancies.
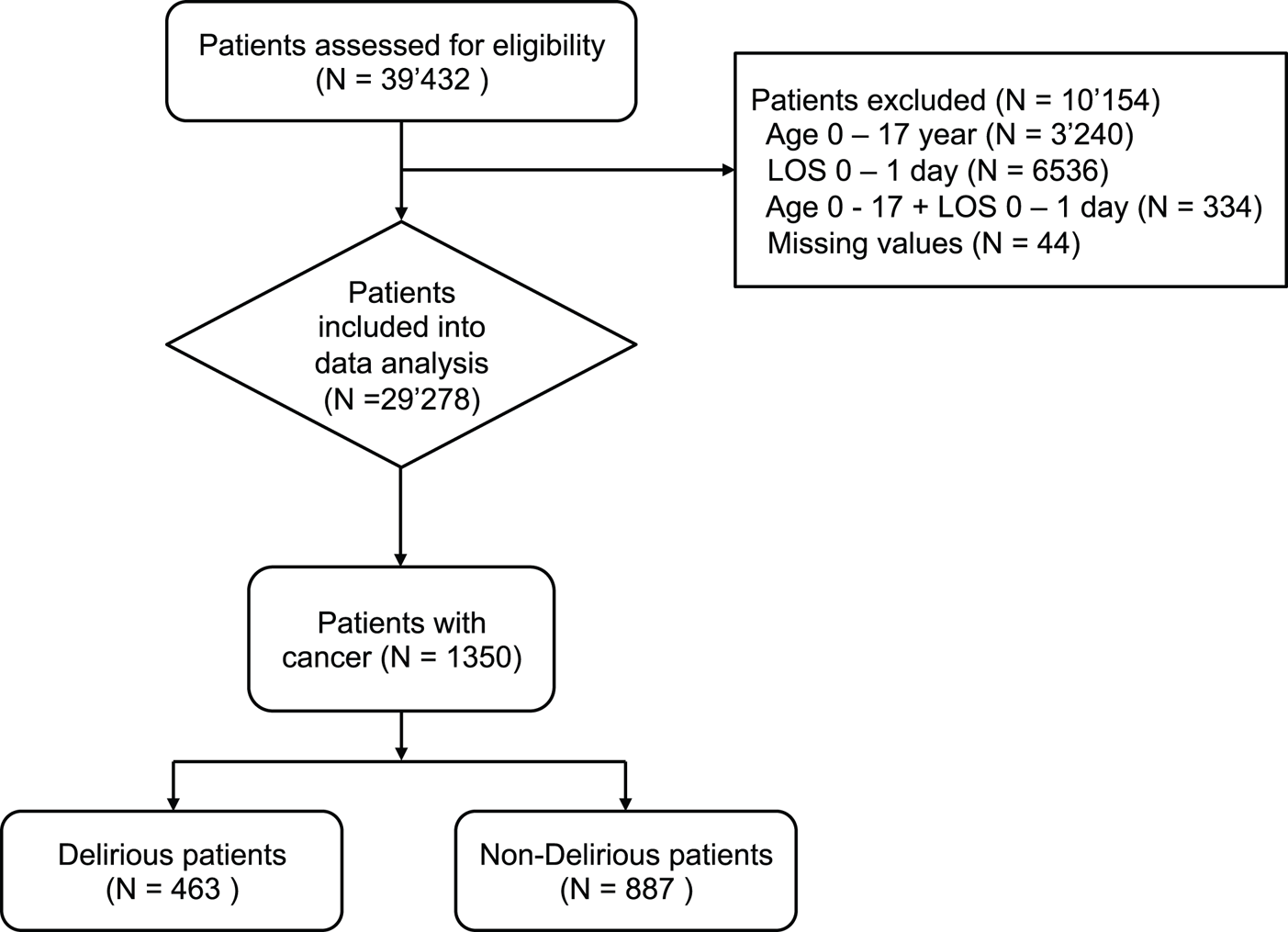
Fig. 1. Screening algorithm for the Delir-Path.
Demographic and medical information was retrieved via the electronic medical chart (Klinikinformationssystem, KISIM, CisTec AG, Zurich). All study procedures performed were in accordance with the World Health Organization's Declaration of Helsinki. The study was reviewed and approved by the Ethics Committee of the Canton Zurich (KEK), Switzerland (KEK-ZH-Nr. 2012-0263). In accordance with the Declaration of Helsinki, Article 29, a waiver of consent was granted by the ethics committee because the majority of our studied population was physically and mentally unable to give consent due to delirium. However, the condition “delirium” (i.e., acute confusional states) that causes incapacity was a necessary characteristic of our research population (World Medical Association, 1964; amended, 2013). This prospective observational study was considered to produce not more than a minimal risk to the study subjects and involved no procedure for which written informed consent was required. Data were collected and reported in accordance with guidelines set by the STROBE (strengthening the reporting of observational studies in epidemiology) statement (STROBE, 2009).
Determination of delirium
The assessment of delirium was based on the DOS scale, the delirium construct as described in the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM-V) consisting of “alertness or inattention and cognitive impairment” (European Delirium Association and American Delirium Society, 2014), and the daily nursing assessment electronic Patient Assessment-Acute Care instrument (ePA-AC) (Hunstein et al., Reference Hunstein, Sippel and Rode2012). This construct accurately identified 97% of the patients diagnosed with delirium according to the DSM-IV-TR (Seiler et al., Reference Seiler, Schubert and Hertler2019). The DOS has been translated into German and has excellent psychometric properties (Bergjan et al., Reference Bergjan, Zilezinski and Schwalbach2020).
In this study, clinical features of delirium were only evaluated cross-sectionally. The DOS was routinely administered three times daily to all patients ≥ 65 years and to patients younger than 65 years with suspected symptoms of incident delirium. Furthermore, various medical and functional parameters were assessed daily by nurses by means of the ePA-AC (Hunstein et al., Reference Hunstein, Sippel and Rode2012). The training of nursing staff included a 4-h session course with mandatory preceding eLearning, case-reports, and state-of-the-art lectures on epidemiology, diagnostic criteria, and screening strategies for delirium, as well as a practical training to apply the DOS tool.
Characterization of predisposing and precipitating factors for delirium in cancer patients
For the purpose of this study, the characterization of the predisposing and precipitating factors for delirium in cancer patients was based upon the formation of diagnostic clusters, according to the 10th revision of the International Statistical Classification of Diseases (ICD-10) (World Health Organization, 1992) and related health problems (Table 1). Frailty is a common syndrome in palliative care patients and characterized by a decline in physiological functioning, reduced strength and endurance, and impaired mobility (Moorhouse and Rockwood, Reference Moorhouse and Rockwood2012). For the purposes of this study, frailty was assessed across the component “mobility” (impaired vs. not impaired) (Seiler et al., Reference Seiler, Schubert and Hertler2019).
Table 1. Diagnostic clusters with their respective included diagnoses according to the ICD-10 and related health problems

Measures
The DOS scale
The 13-item DOS scale was used to screen for delirium (Schuurmans et al., Reference Schuurmans, Shortridge-Baggett and Duursma2003). The DOS scale is a well-validated screening tool for delirium and delirium severity (Schuurmans et al., Reference Schuurmans, Shortridge-Baggett and Duursma2003) based on the DSM-IV (Diagnostic and Statistical Manual of Mental Disorders 4th Edition) delirium criteria (American Psychiatric Association, 2000). The DOS scale includes the following items: disturbances of consciousness (1), attention (2–4), thought processes (5 and 6), orientation (7 and 8), memory (9), psychomotor behavior (10, 11, and 13), and affect (12). Each item is rated as normal (0) or abnormal (1). Items were aggregated throughout recordings; any score ≥ 3 indicates delirium (Gemert van and Schuurmans, Reference Gemert van and Schuurmans2007).
The Charlson Comorbidity Index
To assess multimorbidity, the Charlson Comorbidity Index (CCI) was applied (Charlson et al., Reference Charlson, Pompei and Ales1987). The CCI aggregates multiple medical conditions, including age, myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes mellitus, hemiplegia or paraplegia, renal disease, malignancy, and AIDS/HIV. The medical conditions are rated on a scale from 1 to 6. A total comorbidity score can be computed from the weighted conditions. The CCI shows good reliability and is strongly correlated with mortality and progression-free survival (Williams et al., Reference Williams, Mackenzie and Magnuson2016).
Statistical methods
All analyses were performed with the Statistical Package for the Social Sciences SPSS 26.0 software (IBM Corp., Released, 2017) as well as with R Statistical Software (R Core Team, 2017). Descriptive statistics were reported as means/standard deviations or medians, interquartile ranges or as counts and percentages, as appropriate. All continuous data were tested for normality using Shapiro-Wilk's test. Continuous outcomes were compared using Student's t-tests for parametric or Mann–Whitney U tests for non-parametric or non-normally distributed data, respectively, and categorical variables with Pearson's-χ 2 or Fisher's exact test, where appropriate.
In preparation of the evaluation of the predisposing and precipitating factors for delirium, the data were dichotomized according to the presence or absence of delirium. As a first step, simple logistic regression models were utilized to determine effect sizes of sociodemographic and medical characteristics, as well as the prevalence rates for delirium among cancer patients expressed as odds ratios (OR) with 95% confidence intervals (CIs). Subsequently, multiple logistic regression models were used to determine associations between predisposing and precipitating risk factors and delirium in cancer patients. Multiple regression models were computed with their respective ORs and CIs, based on the results of the simple logistic regressions models, by entering variables with a p-value < 0.15. The model was verified with its Cox-Snell's and Nagelkerke's r 2. As a last step, hazard ratios (HR) from Cox proportional-hazards models were used to determine mortality risk associated with delirium. Survival data were available for up to 140 days. As only few events occurred after the period of 60 days, the Cox proportional-hazards model was adjusted and illustrated for the period “time to death < 60 days”. All tests were two-tailed, and a p-value of < 0.05 was considered to indicate statistical significance.
Results
Demographics and medical characteristics
In this cohort of hematological and oncological patients, the prevalence rate of delirium was 34.3%. There were 463 patients with delirium and 887 patients without delirium. Delirious patients were older, predominantly male (OR 1.51), and had more comorbidities. Over the entire cohort, the major underlying malignancy was non-Hodgkin lymphoma with 22%; 30% of the eligible patients suffered from secondary neoplasm.
Patient with lung cancer had a twofold higher risk of developing delirium, and patients with brain neoplasms had an eightfold higher risk of developing delirium. Furthermore, the presence of secondary neoplasms increased the risk of delirium by the factor 2. Delirious patients were more frequently admitted as emergency cases (OR 2.13), had more surgical interventions, required more often intensive care unit treatment (OR 5.11), and were discharged more frequently to another hospital (OR 2.85) or to an assisted living facility (OR 3.22). Patients with delirium had longer hospital stays, twice the care needs, and accounted for almost double the health care costs per case relative to non-delirious patients. Importantly, delirious patients had a ninefold higher risk of in-hospital mortality. No group difference was found for the type of hospital health insurance. Detailed patient demographics and clinical characteristics are summarized in Table 2.
Table 2. Sociodemographic, medical, and neurological characteristics of the delirious versus non-delirious cancer patients
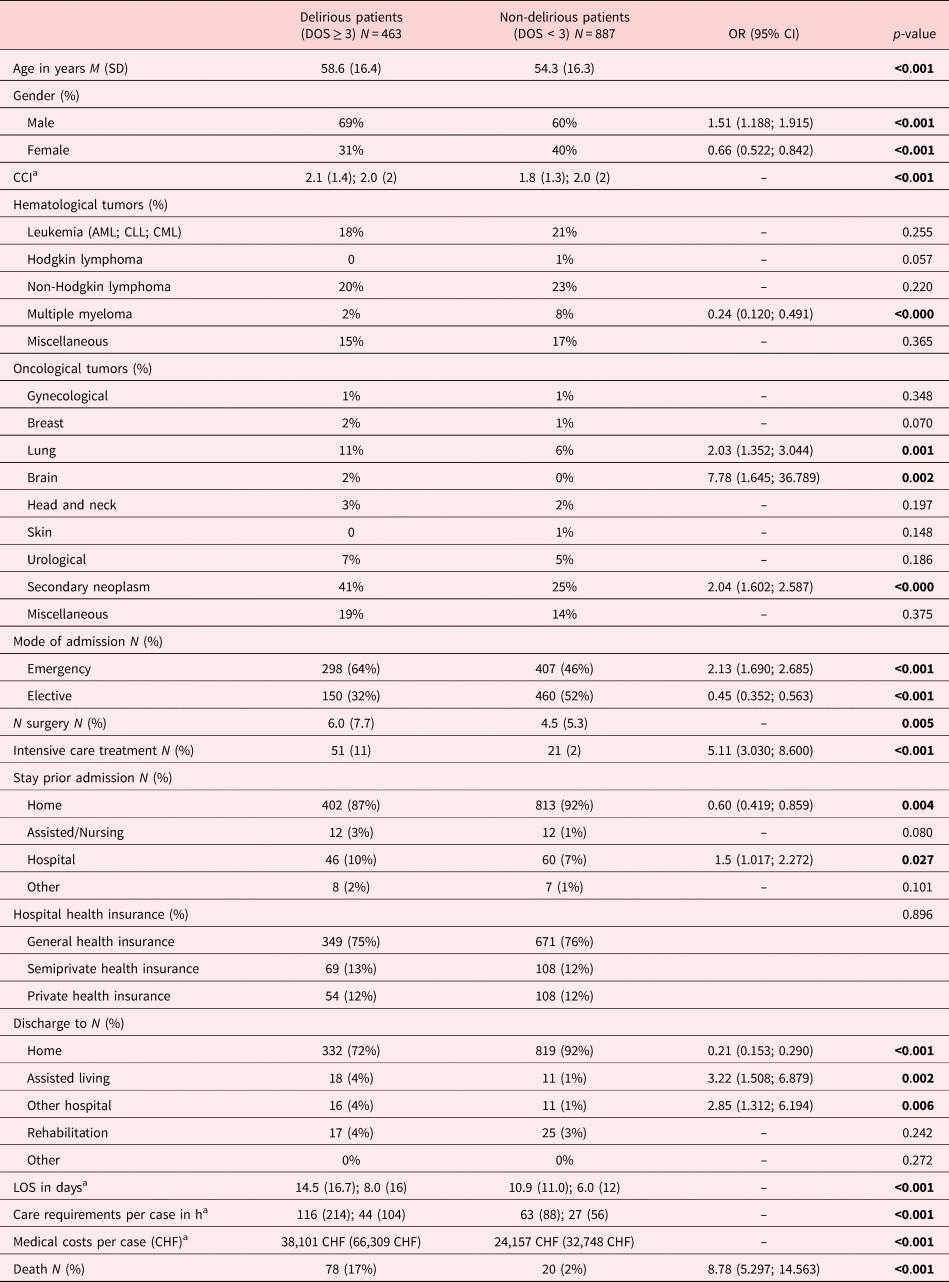
CCI, Charlson Comorbidity Index; M, mean; SD, standard deviation; Md, median; IQR, interquartile range.
Significant levels: significance level was used to determine that they should be in bold. That is, p < 0.05, p < 0.01, p < 0.001.
a Mean, standard deviation; median, interquartile range.
Determination of predisposing and precipitating factors for delirium in hematological and oncological patients
Simple logistic regression identified the following predisposing factors as relevant for delirium: older age (OR 1.71), male gender (OR 1.51), oncological patients (OR 1.73) number of comorbidities (p < 0.001), frailty (OR 31.48), impaired activity of daily living (OR 54.26), hearing and vision impairment (OR 5.24 and 4.14, respectively), the presence of brain neoplasm (OR 13.60), dementia (OR 1.96), epilepsy (OR 2.22), hypertonia (OR 1.81), ischemic heart disease (OR 2.05), cardiac insufficiency (OR 2.25), valvular heart diseases (OR 2.85), acute respiratory distress (OR 0.34), systemic inflammatory response syndrome (SIRS) (OR 2.90), renal insufficiency (OR 2.90), kidney disease (OR 2.90), and thyroid gland diseases (OR 2.32). No significant group differences were observed in terms of neoplastic disease type, inflammatory brain diseases, cardiomyopathy, pulmonary disorders, or substance use disorders.
The most relevant precipitating factors identified by simple logistic regression were sepsis (OR 2.03), acute renal failure (OR 6.27), pressure sores (OR 4.79), cystitis (OR 1.37), and experiencing pain (OR 2.62). No intergroup differences were found for electrolyte imbalances, malnutrition, cachexia, lung edema, or liver failure.
Multiple regression analysis for predisposing and precipitating factors for delirium
Tables 3a and 3b summarize the multiple regression analyses examining the relationship between predisposing and precipitating risk factors and delirium in oncological and hematological patients. On multivariate regression, the most relevant predisposing risk factor was impaired activity with an 11-fold increased risk of delirium. Frailty increased the risk for delirium fivefold, and both impaired hearing and impaired vision increased the risk of delirium twofold. Further predisposing risk factors were chronic pneumonitis (OR 2.61), renal insufficiency (OR 1.81), and hypertension (OR 1.45). No significant associations with delirium in palliative care patients were identified for older age, gender, oncology patients, comorbidities, brain neoplasm, SIRS, thyroid gland diseases, and substance abuse.
Table 3a. Summary of multiple regression models for the predisposing factors for delirium in cancer patients with estimated coefficients (B, SE), 95% CI, and p-values

Cox-Snell and Nagelkerke r 2 = 0.336 and 0.464.
CCI, Charlson Comorbidity Index; SIRS, systemic inflammatory response syndrome; SE, standard error; CI, confidence interval.
Significant levels: significance level was used to determine that they should be in bold. That is, p < 0.05, p < 0.01, p < 0.001.
Table 3b. Summary of multiple regression models for the precipitating factors for delirium in cancer patients with estimated coefficients (B, SE), 95% CI, and p-values

Cox-Snell and Nagelkerke r 2 = 0.054 and 0.074.
SE, standard error; CI, confidence interval.
Significant levels: significance level was used to determine that they should be in bold. That is, p < 0.05, p < 0.01, p < 0.001.
The most relevant precipitating factors for developing delirium were acute renal failure, which increased the risk of delirium by a factor of 8, followed by pressure sores (OR 3.78), the experience of pain (OR 2.86), and cystitis (OR 1.32). Lung edema was not predictive of delirium in palliative care patients. Predisposing and precipitating factors for delirium are depicted in Figure 2.
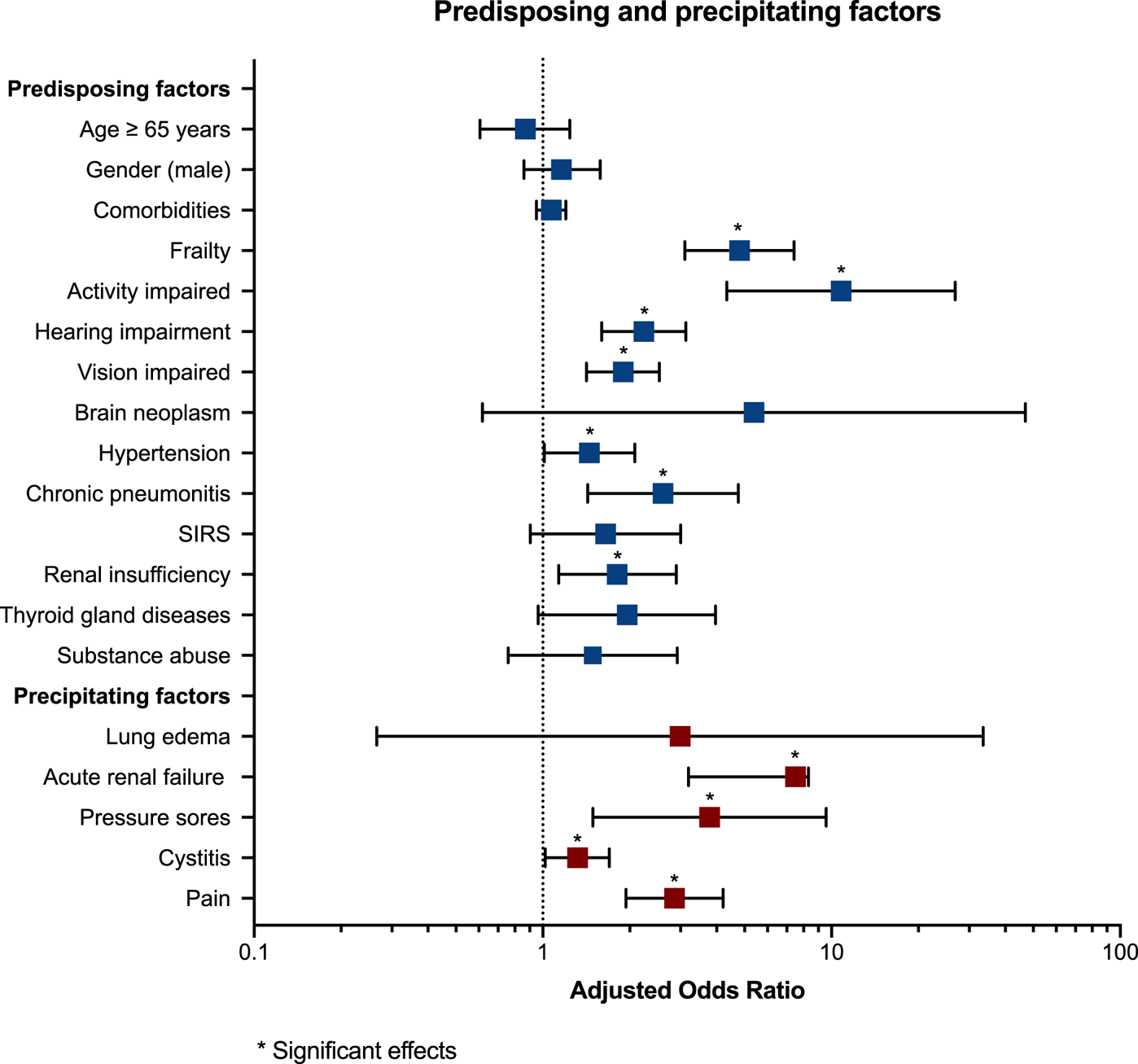
Fig. 2. Forest plots of predisposing and precipitating factors for delirium. OR and 95% CI are reported for each delirium risk factor. The edges of the polygon represent the 95% confidence limit. The graphical representation in the figure refers to the statistics in Table 2.
Cox proportional-hazards model
Results from the Cox proportional-hazards model indicated that delirious patients had a significantly increased risk to die compared with non-delirious patients (the omnibus test of model coefficients, P < 0.001). The presence of delirium in cancer patients increased the mortality risk sixfold, even after adjustment for age, sex, and comorbidities. Furthermore, older age (i.e., ≥ 65 years) and the presence of comorbidities increased the risk of death associated with delirium twofold (Table 4). Figure 3 illustrates HR for death in delirious versus non-delirious patients.
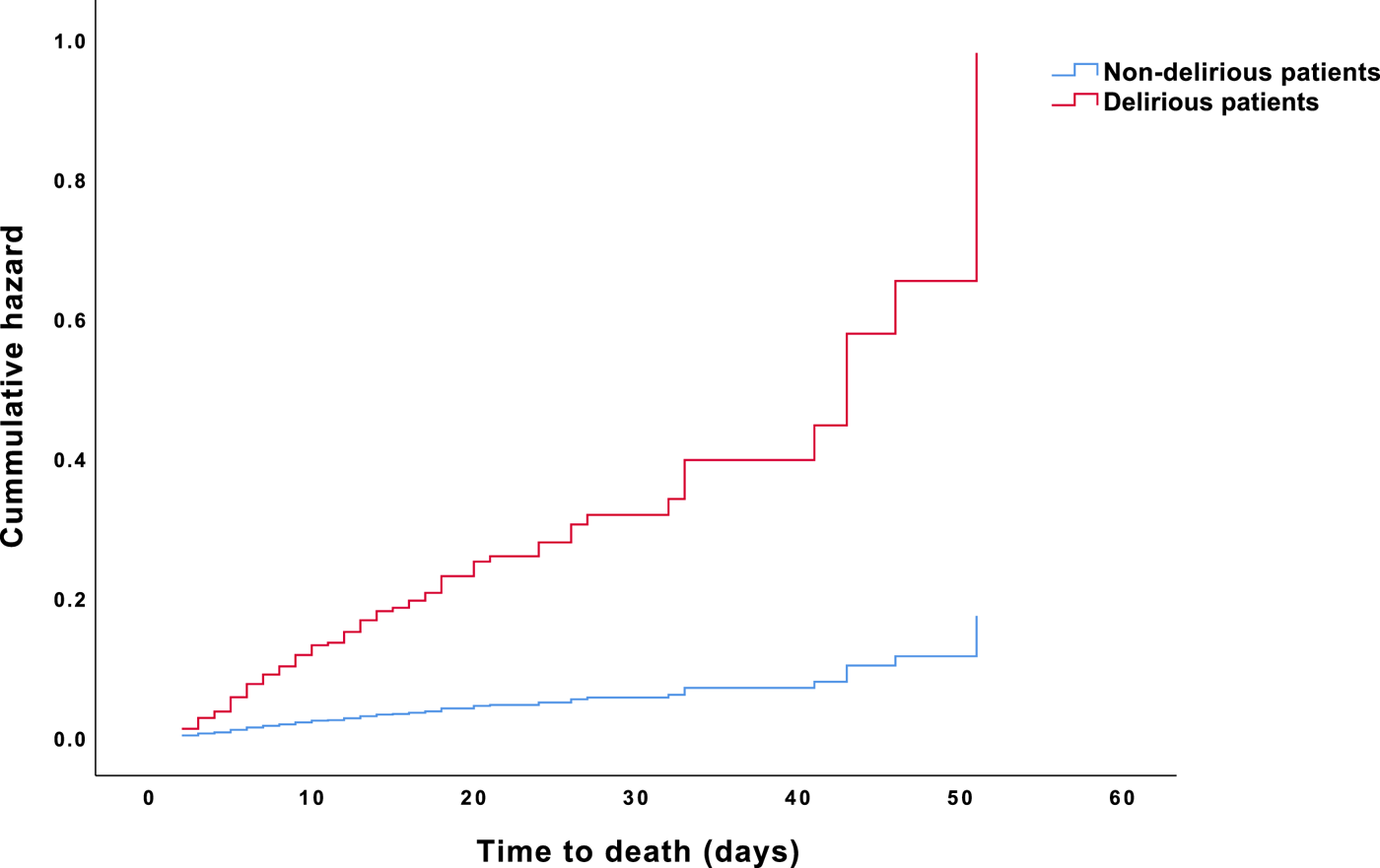
Fig. 3. HR for death in oncological and hematological delirious and non-delirious patients.
Table 4. Cox regression model assessing mortality risk associated with delirium

B, regression coefficient; SE, standard error; HR, hazard ratios; CI, confidence interval; CCI, Charlson Comorbidity Index.
Significant levels: significance level was used to determine that they should be in bold. That is, p < 0.05, p < 0.01, p < 0.001.
Discussion
This prospective study systematically assessed predisposing and precipitating factors for delirium in a large cohort of cancer patients. The prevalence of delirium in this patient cohort was 34%. Frailty, impaired activity, and impaired vision were identified as predisposing risk factors for delirium, as were hypertonia, chronic pneumonitis, and renal insufficiency. Among these predisposing factors, impaired activity and frailty were the most influential risk factors, multiplying the odds of developing delirium by factors 11 and 5, respectively. Meanwhile, acute renal failure, pressure sores, the experience of pain, and cystitis were identified as significant precipitating risk factors. Among these, acute renal failure was the strongest precipitating factor, increasing the odds of developing delirium to almost eight. Moreover, the presence of delirium increased the rate of death to almost six times the rate observed in non-delirious patients, and being 65 years old or older as well as the presence of comorbidities further doubled this risk.
Our results are consistent with previously published results linking delirium in patients with advanced disease to unfavorable short- and long-term outcomes, including increased morbidity and mortality, prolonged hospital stays, increased healthcare requirements and costs, and the need for nursing home placement (Boettger et al., Reference Boettger, Jenewein and Breitbart2015; Schubert et al., Reference Schubert, Schurch and Boettger2018).
In our study, although age, male gender, oncological malignancies, comorbidities, brain metastases, dementia, epilepsy, acute respiratory distress syndrome, SIRS, and sepsis were associated with delirium on univariate analysis, these variables lost their significance in multiple logistic regression models. In the literature, age is considered a robust risk factor for delirium (Inouye et al., Reference Inouye, Westendorp and Saczynski2014). Importantly, the incidence of cancer increases dramatically among individuals aged 65–74 years, mainly due to weakened cellular repair mechanisms (World Health Organization, Reference World Health Organization2018) and cancer is the second leading cause of death in patients 65 years and older (Centers for Disease Control and Prevention (CDC), 2017). Elderly patients also have a high prevalence of other comorbidities, and those with advanced cancer are at particular risk of having delirium precipitated by otherwise relatively innocuous factors like dehydration, infection, bladder catherization, and several medications (e.g., sedatives, hypnotics, and polypharmacy) (Inouye et al., Reference Inouye, Westendorp and Saczynski2014). Contrary to literature findings, in our study, age was not a significant predisposing risk factor for delirium. These results confirm previous findings in a cohort of delirious palliative care patients (Seiler et al., Reference Seiler, Blum and Hertler2020). Furthermore, subgroup analyses revealed no significant differences for the risk of delirium between patients admitted to the oncological versus hematological department. This despite some papers demonstrating that patients with hematologic malignancies might be at higher risk for delirium due to the predominant involvement of the immune system in hematological diseases and aggressive therapies at the end of life (e.g., hematopoietic stem cell transplantation and CAR-T cells therapy), both of which can precipitate delirium (Edwards et al., Reference Edwards, Holmes and Valladarez2016). A possible explanation for this contradictory finding is a relatively high number of admissions of hematological patients to the oncological ward during 2014, which might have biased the results.
On multiple regression analysis, important predisposing factors for delirium included sensory impairments (i.e., hearing and visual impairment), as well as physical frailty. The association between sensory impairment and delirium is well described in the literature. Uncorrected sensory impairment can result in increased delirium rates that subsequently negatively affect clinical outcomes and functional recovery (LaHue and Liu, Reference LaHue and Liu2016). Frailty is typically characterized by diminished strength and endurance and impaired physiological functions, all of which can increase an individual's vulnerability for endogenous and exogenous stressors, adverse health outcomes, disability, and premature death (Clegg et al., Reference Clegg, Young and Iliffe2013). Physical frailty has a particularly strong relationship with delirium in older adults (Williams et al., Reference Williams, Mackenzie and Magnuson2016).
Concordant with previous reports involving different patient cohorts, we identified hypertension, chronic pneumonitis, and renal insufficiency as further major predisposing risk factors for delirium in cancer patients (Oliveira et al., Reference Oliveira, Oliveira and Oliveira2018). Cancer patients are at high risk for hypertension and chronic kidney disease, given that several cancer treatments cause hypertension or renal complications, like acute kidney injury or chronic kidney disease (Rosner and Perazella, Reference Rosner and Perazella2017; Cohen et al., Reference Cohen, Geara and Hogan2019). Furthermore, hypertension can develop indirectly through treatment-related nephrotoxicity (Cohen et al., Reference Cohen, Geara and Hogan2019). Of note, hypertension and renal insufficiency are strongly interconnected and can mutually reinforce each other (Cohen et al., Reference Cohen, Geara and Hogan2019). Moreover, hypertension is one of the most important risk factors for major adverse cardiovascular events in cancer patients (Meijers and de Boer, Reference Meijers and de Boer2019). Pneumonitis is a noninfectious inflammation of the lungs that can develop as an adverse side effect of chemotherapy, radiation, and newer targeted drugs and immunotherapies (Vasiljevic et al., Reference Vasiljevic, Arnold and Neuman2018; Shen et al., Reference Shen, Sheng and Chen2019).
The most important precipitating factor we observed in our patient cohort was acute renal failure, followed by pressure sores, uncontrolled pain, and cystitis. These results corroborate findings in the literature. Commonly reported precipitating factors for delirium include organ failure, infections, electrolyte imbalance, paraneoplastic syndromes, and the side effects of cancer treatment, like acute renal injury (Ljubisavljevic and Kelly, Reference Ljubisavljevic and Kelly2003; Rosner and Perazella, Reference Rosner and Perazella2017).
Particularly notable is the independent association we detected between delirium and increased in-hospital mortality in patients with advanced disease. In our multivariate Cox regression model, patients with delirium were six times as likely to die during their hospitalization as patients without delirium. Older age (≥ 65 years) and the presence of comorbidities as indexed by the CCI were significantly associated with delirium and further doubled the hazard of death. These results support previous conclusions drawn in the literature. In meta-analyses performed by Witlox et al. (Reference Witlox, Eurelings and de Jonghe2010) that enrolled roughly 3,000 elderly patients, delirium was discovered to significantly increase the risk of death — relative to not having delirium — independent of age, sex, comorbidities, illness severity, and baseline dementia (HR 1.95; 95% CI 1.51; 2.52). A similar association was observed in an older study of hospitalized patients, in which patients with delirium exhibited a 13-fold increase in risk of death within the 5 years following hospitalization relative to age-matched patients who had not experienced delirium (van Hemert et al., Reference van Hemert, van der Mast and Hengeveld1994). According to the conceptualization of dynamic risk factors for delirium, patients with advanced cancer, and specifically those who are older and/or physically frail, may develop delirium with any precipitating factor, whereas younger patients and patients with less advanced disease may be more resistant (Bush et al., Reference Bush, Lawlor and Ryan2018). Our results add to the existing literature by providing evidence for certain predisposing and precipitating risk factors for delirium in a large cohort of cancer patients.
Possible mechanisms of delirium development
A well-acknowledged model used to determine the risk for delirium in hospitalized patients involves the interaction between predisposing and precipitating factors. With this theory, it is assumed that patients with a high level of baseline vulnerability (i.e., displaying multiple predisposing factors) need fewer precipitating factors to develop delirium than patients with lower baseline vulnerability (Inouye et al., Reference Inouye, Westendorp and Saczynski2014).
Cancer patients have high “baseline vulnerability” to delirium arising from the cancer itself, as well as from treatment-related adverse effects, complications, and comorbidities (Bush et al., Reference Bush, Lawlor and Ryan2018). Ways by which cancer directly causes delirium include metabolic abnormalities, metastatic disease, brain metastases, vascular disorders, and paraneoplastic and autoimmune syndromes that affect the biological milieu, including neurotransmitter, neuroendocrine, and/or neuroinflammatory pathways. This may result in endocrine, metabolic, and electrolyte derangements that precipitate delirium (Stone and DeAngelis, Reference Stone and DeAngelis2016). In addition, cancer patients are exposed to different treatment modalities, including chemotherapy, immunotherapy, surgery, radiation, and stem cell transplantation, but also newer cancer therapies like immune checkpoint inhibitors and T-cell therapies, many of which can directly induce neurologic complications, including delirium (Stone and DeAngelis, Reference Stone and DeAngelis2016). Furthermore, many side effects of anticancer treatment (e.g., nausea and vomiting), and chronic conditions like pain, sleeping difficulties, and anxiety, often are managed with potentially deliriogenic agents (e.g., anticholinergic medications, benzodiazepines, and narcotics) (Alagiakrishnan and Wiens, Reference Alagiakrishnan and Wiens2004).
In our review of the literature and clinical experience, we note that many patients with terminal illness become delirious before they die, particularly during the final 24–48 h (Bush et al., Reference Bush, Tierney and Lawlor2017). Delirium in patients with advanced disease is frequently a pre-terminal event, making it a precursor of impending death (Friedlander et al., Reference Friedlander, Brayman and Breitbart2004). Importantly, the effect of delirium on the likelihood of death cannot be completely separated from that of the terminal illness itself. Delirium in terminally ill patients is a condition of significant physiological disruption, typically multifactorial in etiology, including organ failure, infections, adverse drug side effects and, in a small percentage, some paraneoplastic syndrome (Friedlander et al., Reference Friedlander, Brayman and Breitbart2004).
Clinical implications
That delirious cancer patients have a risk of death six times as high as those without emphasizes the need to overcome barriers to early palliative care in medical oncology and hematology. We believe that efforts to enhance and hasten cooperation between physicians and palliative care specialists are critical to addressing the multi-dimensional needs of patients with serious illness and their families, to improving quality of care, and to reducing healthcare costs (Schlick and Bentrem, Reference Schlick and Bentrem2019).
Future directions
Tremendous diversity can be found in the literature on precipitating and predisposing factors for delirium in cancer patients, including inconsistent results presumably reflecting the multifactorial underlying causes of delirium (Kang et al., Reference Kang, Shin and Bruera2013; Bush et al., Reference Bush, Lawlor and Ryan2018). Study results also may be affected by study design, the study outcomes evaluated, and the duration of follow-up (Witlox et al., Reference Witlox, Eurelings and de Jonghe2010). In order to address these research problems, there is a pressing need to implement longitudinal randomized controlled trials that can take account of confounding variables, including age, comorbidities, tumor's stage, and tumor site in order to better understand the pathophysiology of delirium in patients with advanced cancer. Furthermore, prospective cohort studies would better delineate the delirium outcomes of patients with advanced cancer diseases. Given attrition challenges in this vulnerable population, multicenter studies could be key to recruiting patient cohorts large enough to allow for more comprehensive analyses. Furthermore, efforts to educate physicians and nurses on delirium screening, the monitoring of risk factors, preventative strategies, delirium recognition, and both pharmacologic and non-pharmacologic management are imperative to improving patients’ clinical outcomes. Implementing some standardized delirium screening process is an important step toward enhancing the effectiveness of delirium treatment. Combining the DOS score and DSM-5 diagnostic criteria for delirium screening proved to be superior to using the DOS instrument alone or the combined DOS/Intensive Care Delirium Screening Checklist (ICDSC), resulting in improved sensitivity and specificity. Complementing the DOS screening with the DSM-5 criteria for delirium presents an innovative, powerful, and time-efficient delirium screening approach. Continued research is warranted to confirm and validate our findings and to compare the sensitivity and specificity of other delirium screening tools (Velthuijsen et al., Reference Velthuijsen, Zwakhalen and Warnier2016).
Strengths and limitations
Important strengths of our study include its rigorous methodology, which include the systematic and comprehensive assessment of precipitating and predisposing risk factors for delirium in a vulnerable patient cohort, and large sample size. A further strength is our utilization of an innovative delirium screening approach, combining the DOS score and DSM-5 diagnostic criteria for delirium. On the other hand, our study had several limitations. First, data collection was limited to a single center, which may limit the extrapolation of results to the general population. Second, certain uncommon factors that might induce delirium, like endocrine disorders and hypoxia, were not included, and brain imaging was not routinely performed. Third, clinical features of delirium were only assessed cross-sectionally; thus, the temporal relation between risk factors and the onset of delirium could not be clarified. Finally, for some risk factors, the CI for the corresponding OR were large, due to the small number of patients with these risk factors; such results should be interpreted with greater caution.
Conclusions
Delirium is a prevalent complication in cancer patients and is associated with significantly increased morbidity and mortality. Our study identified several predisposing and precipitating risk factors for delirium in cancer patients, some of which can be targeted early and modified to reduce symptom burden and improve clinical outcomes. The systemic categorization of predisposing and precipitating factors may suggest new strategies for preventing and managing delirium in cancer patients. Continued research is necessary to confirm our findings and to better understand risk factors for delirium and their potential reversibility, with the ultimate purpose of improving delirium management and clinical outcomes in patients with advanced cancer.
Funding
C.H. obtained funding by the Filling the Gap program, Faculty of Medicine, University of Zurich, Switzerland.
Conflict of interest
The authors have no conflicts of interest to declare.











