Article contents
In vitro compatibility and nematicidal activity of Monacrosporium sinense and Pochonia chlamydosporia for biological control of bovine parasitic nematodes
Published online by Cambridge University Press: 26 April 2021
Abstract
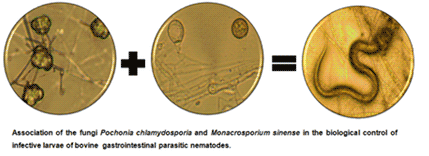
The use of nematophagous fungi is an alternative for the biological control of nematodes in ruminants. In this study, the compatibility of joint growth of the fungi Monacrosporium sinense and Pochonia chlamydosporia and the joint nematicidal activity of these fungal isolates on bovine infective larvae were evaluated. For that, tests of direct confrontation, the effect of volatile compounds and antibiosis were conducted. In order to carry out the tests, the fungi were inoculated in potato dextrose agar culture medium and, after the incubation period, the growth of the colonies, the formation of an inhibition halo and the effect of volatile metabolites were verified. The compatibility between fungi isolates M. sinense and P. chlamydosporia was confirmed and the nematicidal evaluation proved the best effectiveness was when both were used together, with a 98.90% reduction in the number of bovine nematode infective larvae under in vitro conditions. It was concluded that M. sinense and P. chlamydosporia presented synergistic action, suggesting that the joint application of the fungi increases the effectiveness of biological control of bovine infective larvae.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Author(s), 2021. Published by Cambridge University Press
References
- 4
- Cited by



