Published online by Cambridge University Press: 06 December 2021
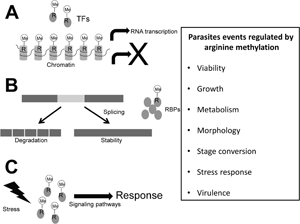
Arginine methylation is a post-translational modification involved in gene transcription, signalling pathways, DNA repair, RNA metabolism and splicing, among others, mechanisms that in protozoa parasites may be involved in pathogenicity-related events. This modification is performed by protein arginine methyltransferases (PRMTs), which according to their products are divided into three main types: type I yields monomethylarginine (MMA) and asymmetric dimethylarginine; type II produces MMA and symmetric dimethylarginine; whereas type III catalyses MMA only. Nine PRMTs (PRMT1 to PRMT9) have been characterized in humans, whereas in protozoa parasites, except for Giardia intestinalis, three to eight PRMTs have been identified, where in each group there are at least two enzymes belonging to type I, the majority with higher similarity to human PRMT1, and one of type II, related to human PRMT5. However, the information on the role of most of these enzymes in the parasites biology is limited so far. Here, current knowledge of PRMTs in protozoan parasites is reviewed; these enzymes participate in the cell growth, stress response, stage transitions and virulence of these microorganisms. Thus, PRMTs are attractive targets for developing new therapeutic strategies against these pathogens.