Introduction
Soil-transmitted helminth (STH) and schistosome infections are 2 major neglected tropical diseases (Carbonell et al., Reference Carbonell, Rodríguez-Alonso, López-Bernús, Almeida, Galindo-Pérez, Velasco-Tirado, Marcos, Pardo-Lledías and Belhassen-García2021) that affect approximately 1.5 billion people, predominantly in low- and middle-income countries (LMICs) (WHO, 2021b). These helminth infections have a considerable public health impact when left untreated with opposing effects on both physical and cognitive development in children and adolescents (Ezeamama et al., Reference Ezeamama, Bustinduy, Nkwata, Martinez, Pabalan, Boivin and King2018; Pabalan et al., Reference Pabalan, Singian, Tabangay, Jarjanazi, Boivin and Ezeamama2018). The diagnosis of STH and schistosome infections in LMICs so far relies mainly on conventional microscopy. With this diagnostic technique, parasite-derived products (including eggs) in stool and urine can be identified and quantified. This technique is laborious, requires well-trained personnel as well as electricity which is often lacking in resource-limited settings (Ajibola et al., Reference Ajibola, Gulumbe, Eze and Obishakin2018; Khurana et al., Reference Khurana, Singh and Mewara2021). Alternatives to conventional microscopy for schistosomiasis diagnosis include the urine-based point-of-care test to detect circulating cathodic antigens and the up-converting particle lateral flow assay to detect circulating anodic antigens in either serum or urine (Corstjens et al., Reference Corstjens, De Dood, Knopp, Clements, Ortu, Umulisa, Ruberanziza, Wittmann, Kariuki, Loverde, Secor, Atkins, Kinung'hi, Binder, Campbell, Colley and Van Dam2020; Hoekstra et al., Reference Hoekstra, Van Dam and Van Lieshout2021). Nucleic acid amplification tests, which involve the detection of parasite's specific nucleic acid sequences, have been used to detect STH and schistosome infections, although mainly in research settings (Obeng et al., Reference Obeng, Aryeetey, De Dood, Amoah, Larbi, Deelder, Yazdanbakhsh, Hartgers, Boakye, Verweij, Van Dam and Van Lieshout2008; Meurs et al., Reference Meurs, Brienen, Mbow, Ochola, Mboup, Karanja, Secor, Polman and Van Lieshout2015; Sanprasert et al., Reference Sanprasert, Kerdkaew, Srirungruang, Charuchaibovorn, Phadungsaksawasdi and Nuchprayoon2019). It is important to note that these existing techniques are still being validated and standardized for use in daily clinical care and medical research in resource-limited settings (Casacuberta-Partal et al., Reference Casacuberta-Partal, Hoekstra, Kornelis, Van Lieshout and Van Dam2019; Amoah et al., Reference Amoah, Hoekstra, Casacuberta-Partal, Coffeng, Corstjens, Greco, Van Lieshout, Lim, Markwalter, Odiere, Reinhard-Rupp, Roestenberg, Stothard, Tchuem Tchuenté, De Vlas and Van Dam2020; Cools et al., Reference Cools, Van Lieshout, Koelewijn, Addiss, Ajjampur, Ayana, Bradbury, Cantera, Dana, Fischer, Imtiaz, Kabagenyi, Lok, Mccarthy, Mejia, Mekonnen, Njenga, Othman, Shao, Traub, Van Esbroeck, Vercruysse, Vlaminck, Williams, Verweij, Van Hellemond and Levecke2020; Cools et al., Reference Cools, Vlaminck, Verweij and Levecke2021; Corstjens et al., Reference Corstjens, De Dood, Knopp, Clements, Ortu, Umulisa, Ruberanziza, Wittmann, Kariuki, Loverde, Secor, Atkins, Kinung'hi, Binder, Campbell, Colley and Van Dam2020).
To accelerate control and elimination of STH and schistosome infections, focus is placed mainly on mass drug administration (MDA) of praziquantel prioritizing school age children. In addition, approaches such as increased access to clean water and sanitation, snail control, education and behavioural changes are adopted, together with a multi-diagnostic approach to support monitoring and evaluation of these programmes (Raso et al., Reference Raso, Essé, Dongo, Ouattara, Zouzou, Hürlimann, Koffi, Coulibaly, Mahan, Yapi, Koné, Coulibaly, Meïté, Guéhi-Kabran, Bonfoh, N'goran and Utzinger2018). However, due to the limitations of the existing diagnostic tools needed to monitor, evaluate and guide these programmes, the WHO developed target product profiles (TPPs) to guide development of new diagnostics to reliably measure the effectiveness of these programmes (WHO, 2021a, 2021b). Therefore, new reliable diagnostic tools, designed to match the WHO TPP, are required for effective disease control and elimination. Automated or semi-automated easy-to-use optical devices for the detection of parasite eggs in feces or urine, and based on low-cost platforms may provide a matching solution.
Innovative optical devices, designed to (semi-) automatically detect helminth eggs, have been built based on technical modifications of the optical train of a smartphone. The integrated sensors are commonly used for image acquisitions in such systems (Hernández-Neuta et al., Reference Hernández-Neuta, Neumann, Brightmeyer, Ba Tis, Madaboosi, Wei, Ozcan and Nilsson2019; Vasiman et al., Reference Vasiman, Stothard and Bogoch2019). Another optical technique is based on the direct coupling of the smartphone to the ocular of a standard microscope to digitally register the images of the sample under examination (Yang et al., Reference Yang, Bakhtari, Langdon-Embry, Redwood, Lapierre, Rakotomanga, Rafalimanantsoa, Santos, Vigan-Womas and Knoblauch2019; Dacal et al., Reference Dacal, Bermejo-Peláez, Lin, Álamo, Cuadrado, Martínez, Mousa, Postigo, Soto, Sukosd, Vladimirov, Mwandawiro, Gichuki, Williams, Muñoz, Kepha and Luengo-Oroz2021). Increased processing power of the smartphones has equally been exploited for the development of artificial intelligence (AI) algorithms. The AI algorithms are developed for interpretation and analysis of registered data. Such devices have been reported to possess the potential for automated medical diagnosis (Saeed and Jabbar, Reference Saeed and Jabbar2018; Vasiman et al., Reference Vasiman, Stothard and Bogoch2019). Over the last years, several investigators have reported the use of such devices to detect STHs and schistosome infections. In these studies, high-quality images from samples were acquired and analysed for the presence/absence of these parasite eggs. The readiness of these devices in LIMCs can be determined using the technology readiness level (TRL) developed by the National Aeronautics and Space Administration (NASA) and implemented by the European Commission (EC) (Bruno et al., Reference Bruno, Lobo, Covino, Donarelli, Marchetti, Panni and Molinari2020). However, the TRL scale does not consider extensive medical and design outcomes.
To better understand the progress made so far in the development of these optical devices and the barriers that limit transitions to field applications, we examined publications in peer-reviewed journals with a specific focus on the validation of innovative optical devices (hardware with or without AI) used for the detection and/or quantification of eggs of STHs and Schistosoma spp. in human urine and stool samples. We also highlight the progress made in the development of optical devices with AI-assisted detection. Furthermore, we adapted the TRL (combined TRL-TPP) scale and used it to qualify readiness of innovative optical devices developed for LIMCs to diagnose soil-transmitted helminthiasis and schistosomiasis. The clinical diagnostic performance, advantages and disadvantages of the use of these devices are also discussed.
Methodology
Bibliography search strategy and article selection
PubMed and Google Scholar were searched following their respective stipulated database guidelines from March 2020 to May 2022 for English language-based literature published in peer-reviewed journals between 2010 and 2022. The focal terms for devices with or without AI (such as, cell phone OR smartphone OR mobile phone OR digital microscopy OR digital imaging OR microscope OR artificial intelligence OR machine learning OR neural network OR deep learning OR computer vision) were combined with diagnostic OR diagnosis using the AND Boolean operator and the results of this combination operated with the disease names (e.g. helminthiasis OR ascariasis OR ancylostomiasis OR trichuriasis OR schistosomiasis OR Bilharzia OR soil-transmitted helminth infection) OR pathogen names (e.g. A. lumbricoides OR T. trichiura OR S. haematobium OR S. mansoni OR STH-species) using the AND Boolean operator. In total, 849 hits were found and filtered by scanning the titles for terminologies related to optical devices listed above and parasite names of interest. This resulted in 30 selected articles of which the abstracts were read by the first author and assessed for their relevance to the review. Publications deemed relevant were read in full, and thereafter 16 were selected which focused on innovative optical devices and their assessment for the detection of parasites of interest in human stool and/or urine. In addition, a short report and a conference paper cited within these 16 selected publications were also included, resulting in a total of 18 articles (see Table 1). Publications that focus on AI algorithms for the detection of STHs or Schistosoma spp. without device prototype description were excluded (Cooke et al., Reference Cooke, Laing, White, Wakes and Sowerby2015; Jiménez et al., Reference Jiménez, Maya, Velásquez, Torner, Arambula, Barrios and Velasco2016; Alva et al., Reference Alva, Cangalaya, Quiliano, Krebs, Gilman, Sheen and Zimic2017; Li et al., Reference Li, Li, Liu, He, Wang, Xu, Guan, Chen, Qi and Wang2020; Butploy et al., Reference Butploy, Kanarkard and Maleewong Intapan2021).
Table 1. Overview of the 18 included publications in order of year of publications

KK, Kato–Katz; (–), not mentioned; Sen, sensitivity; Spe, specificity; TPP, target product profile.
Analysis
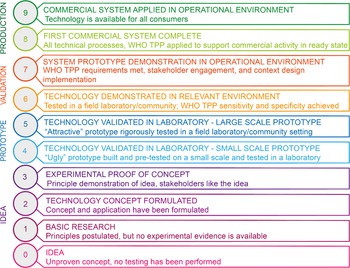
To understand the developmental progress and the performance evaluation process in a conceptual frame, the devices in the various studies were grouped as either smartphone-based optical devices or non-smartphone-based optical devices. Furthermore, each device within these 2 groups was ranked for readiness (for any of the disease-causing parasites; Ascaris lumbricoides, Trichuris trichiura, Schistosoma haematobium, Schistosoma mansoni and hookworm) using an adapted version of the 9-level TRL scale (NASA) (Fig. 1). The TRL was modified by distributing the WHO TPP on levels 6, 7 and 8 (WHO, 2021a, 2021b). That is the recommended sensitivity and specificity on TRL-6 and the other profiles (such as prototype use summary, configuration and context design not excluding diagnostic performance) on TRL-7 and -8 as a requirement to be met to transition to the next level on the scale. Three of the co-authors applied the adapted scale to 21 devices from the 18 selected publications. All information about each device was extracted from the respective study such as aim of the study; image of prototype(s) evaluated; evaluation setting; sample size; an image captured by the device; the TPP evaluated for each device including the diagnostic performance. Where available, additional information on context-specific design through stakeholder engagement and usability was examined. With this information, each expert ranked the devices based on the adapted scale (see Tables 2 and 3). A particular rank (1–9) allocated to a device implies it meets the criteria of the rank and all below it. For devices of different iterations that were validated in different studies, the rank of the most recent iteration (study) was considered to determine its overall progress.

Fig. 1. The 9-scale TRL classification chart. Image adapted from Bruno et al. (Reference Bruno, Lobo, Covino, Donarelli, Marchetti, Panni and Molinari2020). TPP, target product profile.
Table 2. Diagnostic characteristics of 11 smartphone-based optical devices for the diagnosis of Schistosoma and STH infections

TRL, technology readiness level scale; (–), not stated; CI, confidence interval; Sen, sensitivity; Spe, specificity; PPV, positive predictive value; NPV, negative predictive value. (*) See Fig. 1 for TRL specification.
Superscript KK, SSTT, UF=Kato–Katz, spontaneous sedimentation and filtration technique slides read by a microscopist.
Superscript AI=Kato–Katz, spontaneous sedimentation and filtration technique slides analysed by artificial intelligence (AI) algorithm.
Table 3. Diagnostic characteristics of 10 non-smartphone-based optical devices for diagnosis of Schistosoma and STH infections

TRL, technology readiness level scale; (–), not stated; CI, confidence interval; Sen, sensitivity; Spe, specificity; PPV, positive predictive value; NPV, negative predictive value. (*) See Fig. 1 for TRL specification.
Evolution of optical device and performance
Innovative optical devices have evolved over the years based on system designs, and rapid advances in the technological development of integrated functional parts such as general processors, image registration sensors, etc. For simplicity, all the optical devices discussed in this study are grouped into 2 main categories: (1) smartphone-based devices and (2) non-smartphone-based devices.
Smartphone-based optical devices
Modification of the functionalities of smartphones allows for the rapid development of smartphone-based optical devices to visualize and quantify parasite-derived morphological products in samples. Their portability makes them suitable for parasitology field surveys. In the past decade, numerous studies have been carried out using smartphone-based optical devices of which some are limited to proof-of-concept while others have been validated through a field evaluation (Bogoch et al., Reference Bogoch, Andrews, Speich, Utzinger, Ame, Ali and Keiser2013; Bogoch et al., Reference Bogoch, Coulibaly, Andrews, Speich, Keiser, Stothard, N'goran and Utzinger2014b; Switz et al., Reference Switz, D'ambrosio and Fletcher2014; Ephraim et al., Reference Ephraim, Duah, Cybulski, Prakash, D'ambrosio, Fletcher, Keiser, Andrews and Bogoch2015; Coulibaly et al., Reference Coulibaly, Ouattara, D'ambrosio, Fletcher, Keiser, Utzinger, N'goran, Andrews and Bogoch2016b; Sowerby et al., Reference Sowerby, Crump, Johnstone, Krause and Hill2016; Bogoch et al., Reference Bogoch, Koydemir, Tseng, Ephraim, Duah, Tee, Andrews and Ozcan2017; Koydemir et al., Reference Koydemir, Coulibaly, Tseng, Bogoch and Ozcan2019; Yang et al., Reference Yang, Bakhtari, Langdon-Embry, Redwood, Lapierre, Rakotomanga, Rafalimanantsoa, Santos, Vigan-Womas and Knoblauch2019; Armstrong et al., Reference Armstrong, Harris, D'ambrosio, Coulibaly, Essien-Baidoo, Ephraim, Andrews, Bogoch and Fletcher2022). Table 2 recapitulates the diagnostic characteristics of smartphone-based optical devices for the detection of STHs and Schistosoma spp. and the corresponding TRL assigned by the authors of this review.
In 4 published studies, 5 smartphone-based optical devices for the detection of helminth eggs in urine and/or stool were reported as experimental proof-of-concept studies (Bogoch et al., Reference Bogoch, Andrews, Speich, Utzinger, Ame, Ali and Keiser2013; Switz et al., Reference Switz, D'ambrosio and Fletcher2014; Sowerby et al., Reference Sowerby, Crump, Johnstone, Krause and Hill2016; Yang et al., Reference Yang, Bakhtari, Langdon-Embry, Redwood, Lapierre, Rakotomanga, Rafalimanantsoa, Santos, Vigan-Womas and Knoblauch2019). First, Bogoch et al. carried out a study within an ongoing clinical trial from which 199 Kato–Katz (KK) thick smears were analysed for the presence of STH eggs using a smartphone-based microscope with conventional light microscopy as a reference (Bogoch et al., Reference Bogoch, Andrews, Speich, Utzinger, Ame, Ali and Keiser2013). The device used for this study consisted of a 3 mm ball lens that was mounted in alignment with the lens of an iPhone 4S using a double-sided tape. The light source was a separate handheld incandescent lamp powered by 1 AA battery. For this design, a moderate sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for the detection of STH eggs were observed (Table 2). Compared to conventional light microscopy, the authors observed a higher sensitivity of 93.3% (66.0–99.7) for moderate and high infection intensities of A. lumbricoides. According to the authors, this device is a promising point-of-care diagnostic tool for helminthic infections. However, this design lacks a functional sample stage which makes slide scanning unfeasible in practice.
Second, Switz et al. carried out a proof-of-concept study using a smartphone microscope to image the eggs of A. lumbricoides in stool samples over a wide field of view (FOV) (Switz et al., Reference Switz, D'ambrosio and Fletcher2014). The smartphone microscope consisted of an iPhone 4S coupled to a reversed iPhone 4S lens and embodied by a frame with a screw-based focusing system, an x–y stage and a light-emitting diode (LED) light source. The system set-up provided high-resolution images with significantly large FOV. While the authors conclude that the availability and use of this device in resource-limited settings will increase, its robustness and diagnostic performance at patient level are still unknown and require more testing.
Third, Sowerby et al. carried out a proof-of-concept study that uses the flotation technique to accumulate A. lumbricoides ova in a single FOV for imaging using a smartphone-based microscope (Sowerby et al., Reference Sowerby, Crump, Johnstone, Krause and Hill2016). Stool was collected from a person known to be positive for ascariasis. The smartphone-based microscope consisted of a Nokia Lumia 1020 smartphone, an external double convex objective lens, a cassette containing a sample well and a cassette rack with a light source – all coupled together such that the sample well, external lens and the lens of the smartphone are aligned. Several images of a single FOV at different focal lengths were captured for image reconstruction and visualization of A. lumbricoides eggs. According to the authors this device has potential as a point-of-care device for the detection of helminth species upon further development. The sample preparation method and the processing of images through reconstruction clearly do not support the design as a point-of-care test (Peeling and Mabey, Reference Peeling and Mabey2010; Osredkar, Reference Osredkar2017), especially with stool material. Furthermore, functionalities of point-of-care devices were not examined for this prototype.
The fourth proof-of-concept by Yang et al. focused on the development and evaluation of a smartphone-based microscope with and without applying the Kankanet AI algorithm, for the detection of A. lumbricoides, T. trichiura and hookworm eggs. KK, spontaneous sedimentation technique in tube, and merthiolate–iodine–formaldehyde slides were analysed with standard microscopy as a reference (Yang et al., Reference Yang, Bakhtari, Langdon-Embry, Redwood, Lapierre, Rakotomanga, Rafalimanantsoa, Santos, Vigan-Womas and Knoblauch2019). This study was embedded in an ongoing epidemiology survey. The smartphone based-microscope setup consisted of a smartphone mounted on a tripod and connected to a USB video class microscope mounted on a translational stage that moves in the x–y direction. The number of samples included in the study was not stated and evaluation of the device prototype with Kankanet was based on the detection of eggs at an image level. Sensitivity and specificity were determined based on 124 images captured with the device setup as shown in Table 2. The Kankanet AI yielded a lower sensitivity and specificity for the detection of these parasite eggs compared to the microscopist (see Table 2). According to the authors, the setup with the Kankanet AI, if further developed, can achieve a performance comparable to microscopy, and the device could serve as a point-of-care diagnostic procedure. To support this claim, a more thorough performance evaluation of this tool should be carried out at a sample level.
In addition to the aforementioned proof-of-concept studies, 5 different smartphone-based optical devices were field validated for the detection of helminth eggs in urine and/or stool with 2 of the devices evaluated twice in different settings (Bogoch et al., Reference Bogoch, Coulibaly, Andrews, Speich, Keiser, Stothard, N'goran and Utzinger2014b; Ephraim et al., Reference Ephraim, Duah, Cybulski, Prakash, D'ambrosio, Fletcher, Keiser, Andrews and Bogoch2015; Coulibaly et al., Reference Coulibaly, Ouattara, D'ambrosio, Fletcher, Keiser, Utzinger, N'goran, Andrews and Bogoch2016b; Bogoch et al., Reference Bogoch, Koydemir, Tseng, Ephraim, Duah, Tee, Andrews and Ozcan2017; Koydemir et al., Reference Koydemir, Coulibaly, Tseng, Bogoch and Ozcan2019; Armstrong et al., Reference Armstrong, Harris, D'ambrosio, Coulibaly, Essien-Baidoo, Ephraim, Andrews, Bogoch and Fletcher2022). First, a cohort validation study by Bogoch et al. was integrated into an ongoing study in South Côte d'Ivoire. In this study, a single KK thick smear per sample from a total of 164 stool samples were examined with a smartphone-based microscope to detect the eggs of T. trichiura and S. mansoni, with conventional light microscopy as the reference (Bogoch et al., Reference Bogoch, Coulibaly, Andrews, Speich, Keiser, Stothard, N'goran and Utzinger2014b). The smartphone-based microscope consisted of a 3 mm glass ball lens mounted on an S4 iPhone with the support of 1/16 thick foam tape. The sensitivity, specificity, PPV and NPV were reported as shown in Table 2. The authors found this device not ready for use in clinical or epidemiological settings due to its low sensitivity.
The second validation study, carried out by Ephraim et al., was embedded within an ongoing epidemiology survey of schistosomiasis and soil-transmitted helminthiasis in the Central Region of Ghana. A total of 49 participants were randomly selected to provide urine samples for examination of S. haematobium eggs using 2 devices, a smartphone-mounted Foldscope and a reversed-lens CellScope, with conventional microscopy as the reference (Ephraim et al., Reference Ephraim, Duah, Cybulski, Prakash, D'ambrosio, Fletcher, Keiser, Andrews and Bogoch2015). Both devices were iPhone S5-based. However, the Foldscope consisted of an LED light source attached to the phone lens with the help of a paper-based attachment, a magnet and tape, whereas the CellScope consisted of a 3D-printed plastic embodiment with an integrated lens, fixed to the iPhone's lens. The urine samples (10 mL) were processed by centrifugation and examined by both devices and conventional light microscopy. The sensitivity and specificity of the smartphone-mounted Foldscope and the reversed-lens CellScope are shown in Table 2. The authors concluded that these devices could be of use in epidemiological and clinical settings upon further technical adjustments. The design prototype reported in this study did not have a sample stage without which its application in epidemiological surveys for mass disease screening is not feasible. Coulibaly et al. further evaluated the performance of the CellScope in a cross-sectional exploratory study for the diagnosis of schistosomiasis, with conventional light microscopy as a reference (Coulibaly et al., Reference Coulibaly, Ouattara, D'ambrosio, Fletcher, Keiser, Utzinger, N'goran, Andrews and Bogoch2016b). This study examined filtered urine slides and single KK thick smears from 226 participants with sensitivity and specificity reported in Table 2. The sensitivity of the CellScope was lower in low infection intensity samples (<40 eggs 10 mL−1) and strongly correlated with light microscopy in terms of S. haematobium egg count estimated with a Pearson's correlation coefficient of 0.92. Based on the outcome, the authors concluded that this device may be of use in areas with moderate and high intensity of infection. However, the lack of a sample stage makes diagnosis with this prototype impractical.
The fourth validation study also by Bogoch et al. explored an ongoing epidemiological survey, of which 60 urine specimens were randomly selected and examined for S. haematobium eggs using a smartphone-based microscope with conventional microscopy-based centrifugation as the reference standard (Bogoch et al., Reference Bogoch, Koydemir, Tseng, Ephraim, Duah, Tee, Andrews and Ozcan2017). The device was a custom-made 3D-printed optomechanical attachment with an inbuilt lens mounted in alignment with the lens of a Nokia Lumia 1020 smartphone. It also contained 2 LED light sources, 2 polymer diffusers and an x–y stage for manual adjustment of sample slides. The sensitivity, specificity, PPV and NPV for the detection of S. haematobium are shown in Table 2. The sensitivity of the device when separately analysed for low- and high-intensity infections was 65.7% (95% CI 47.7–80.3) and 100.0% (95% CI 59.8–100.0), respectively. Based on these findings, the authors suggested this prototype for use in high infection intensity settings. Similarly, Koydemir et al. evaluated the smartphone-based microscope prototype previously validated by Bogoch et al. (Reference Bogoch, Koydemir, Tseng, Ephraim, Duah, Tee, Andrews and Ozcan2017) in an ongoing epidemiology study in a different setting. Urine and KK thick smear slides were imaged for manual identification of S. mansoni and S. haematobium eggs and detection was at an image level (Koydemir et al., Reference Koydemir, Coulibaly, Tseng, Bogoch and Ozcan2019). The number of urine and stool samples used in this study was not stated. The authors concluded that, this device may be a valuable diagnostic tool in endemic settings only after carrying out rigorous field validation. However, a pilot study to show promising diagnostic performance of this prototype at patient level would concretize this conclusion.
The sixth study by Armstrong et al. evaluated the SchistoScope; a modified version of the LoaScope [developed previously to detect Loa loa microfilaria (Kamgno et al., Reference Kamgno, Pion, Chesnais, Bakalar, D'ambrosio, Mackenzie, Nana-Djeunga, Gounoue-Kamkumo, Njitchouang, Nwane, Tchatchueng-Mbouga, Wanji, Stolk, Fletcher, Klion, Nutman and Boussinesq2017)] as a point-of-care device to automate quantitative detection of S. haematobium eggs with microscopy as a reference (Armstrong et al., Reference Armstrong, Harris, D'ambrosio, Coulibaly, Essien-Baidoo, Ephraim, Andrews, Bogoch and Fletcher2022). The prototype consists of an iPhone 8 with a reverse lens technology (previously described in this section) and a specialized cartridge to concentrate S. haematobium eggs in a single FOV. A total of 205 urine samples were collected to determine the sensitivity and specificity of the SchistoScope with AI and manual detection (Table 3). The authors concluded that this tool has a potential to provide quantitative diagnosis of S. haematobium. However, the cartridge used in this study for sample preparation resulted in approximately 79% of eggs lost during the proposed sample preparation method and therefore requires improvements.
Non-smartphone-based optical devices
Of the 8 non-smartphone-based optical devices reviewed here, six were limited to proof-of-concept (Linder et al., Reference Linder, Grote, Varjo, Linder, Lebbad, Lundin, Diwan, Hannuksela and Lundin2013; Holmstrom et al., Reference Holmstrom, Linder, Ngasala, Martensson, Linder, Lundin, Moilanen, Suutala, Diwan and Lundin2017; Sukas et al., Reference Sukas, Van Dorst, Kryj, Lagatie, De Malsche and Stuyver2019; Agbana et al., Reference Agbana, Nijman, Hoeber, Van Grootheest, Van Diepen, Van Lieshout, Diehl, Verhaegen, Vdovine and Coté2020; Dacal et al., Reference Dacal, Bermejo-Peláez, Lin, Álamo, Cuadrado, Martínez, Mousa, Postigo, Soto, Sukosd, Vladimirov, Mwandawiro, Gichuki, Williams, Muñoz, Kepha and Luengo-Oroz2021; Oyibo et al., Reference Oyibo, Jujjavarapu, Meulah, Agbana, Braakman, Van Diepen, Bengtson, Van Lieshout, Oyibo, Vdovine and Diehl2022) as reported by the authors. The first study by Linder et al. demonstrated the use of a mini-microscope (On-Chip) to image and detect the eggs of S. haematobium with AI (Linder et al., Reference Linder, Grote, Varjo, Linder, Lebbad, Lundin, Diwan, Hannuksela and Lundin2013). In this study, pooled urine samples from a pre-existing quality and assurance survey were used. The mini-microscope consisted of a webcam image sensor chip and an LED as a light source, all connected to computer software for image capture. Two AI algorithms were deployed for the detection of S. haematobium eggs in urine-captured images (see Table 3). Stool samples were also imaged, and eggs of T. trichiura and S. mansoni were morphologically distinguished by a technician. Based on the outcome, the authors concluded that this setup could be exploited for building simpler imaging low-cost devices for diagnosis of schistosomiasis and STHs. However, in this study, automated detection was applied for urine parasite detection only, and not for stool parasites.
The second study by Holstrom et al. reported the imaging performance of a digital microscope and a deep learning-based image analysis algorithm, with human experts as a comparator, for the identification of helminth eggs in urine and stool samples (Holmstrom et al., Reference Holmstrom, Linder, Ngasala, Martensson, Linder, Lundin, Moilanen, Suutala, Diwan and Lundin2017). The design of the digital microscope included a white LED with a bandpass filter and an ultraviolet LED to support both bright field and fluorescent imaging. This digital microscope was constructed from mobile phone-based camera components, such as an external motor unit attached for precise manual adjustment of the glass slide, and it was connected to a laptop with the AI algorithm for image processing. Sixteen positive stool slides and 5 positive urine slides were prepared, and 7385 images were captured with the portable microscope. Of this total number of images captured, 410 were labelled as positive for helminth eggs by human experts and were used for subsequent training and evaluation of the AI algorithm. For detection on an image level for eggs of A. lumbricoides, T. trichiura and hookworm by the algorithm, the sensitivity and PPV were computed (see Table 3). Based on the authors, the device produced sufficient image quality for manual and automatic detection of STH and S. haematobium eggs using a deep learning algorithm. Further validation of this setup at patient level is required before implementation in field settings can be considered.
The third study developed and tested a lab-on-a-disk (LOD) digital platform, to image and manually count STHs and S. mansoni eggs in a single FOV with Mini-FLOTAC and McMaster as reference tests (Sukas et al., Reference Sukas, Van Dorst, Kryj, Lagatie, De Malsche and Stuyver2019). Stool samples were processed by floatation and eggs collected in a single FOV. The complete design included a setup for imaging, a minicentrifuge and a sample disk where the sample is loaded. The imaging setup consisted of a macro lens, an adapter, a 10 × objective lens and a halogen light source. These parts were mounted in that order to a Sony α5100 camera and the imaging zone of the sample disk, in a vertical axis. The Sony camera was connected to a Samsung tablet for the camera control and image capture. Seven positive stool samples were examined using the 3 methods. The Mini-FLOTAC counts correlated with the LOD counts from the whole chamber of the disk (correlation coefficient 0.91). In addition, samples with low infection intensity of STHs (~30–100 egg per gram) determined with Mini-FLOTAC and McMaster were also detected as egg positive by the LOD device but with a lower egg count. According to the authors, this platform produced quality images to properly detect and quantify eggs of parasites in stool. Nonetheless, the sample disk needs further improvement since eggs were lost along the channel path leading to the collection zone where quantification in a single FOV was aimed.
The fourth study by Agbana et al. reported the use of a novel lens-free imaging system in combination with flow cytometry and AI (SODOS) to image and detect S. haematobium eggs in comparison with conventional microscopy (Agbana et al., Reference Agbana, Nijman, Hoeber, Van Grootheest, Van Diepen, Van Lieshout, Diehl, Verhaegen, Vdovine and Coté2020). The SODOS device consists of an imaging sensor, a micro-channel flow cell and a monochromatic light source aligned on the same optical axis. It also contains an automatic urine injection system with a calibrated syringe for pumping the sample into the flow cell and a cup for collecting the outlet of the flow cell. To evaluate the device performance, urine samples collected from uninfected volunteers were spiked with S. haematobium eggs (17 eggs mL−1). Per run, 10.7 mL urine was imaged by the device, followed by image analysis using the AI software programme. An accuracy of 96.8% and a sensitivity and specificity for the AI software programme were determined (see Table 3). According to the authors, the SODOS may serve as a future tool to complement existing techniques for the diagnosis of urinary schistosomiasis if the sensitivity is further improved. Furthermore, this study was limited to spiked urine samples and validation using clinical samples is required.
The study by Dacal et al. reported the use of a mobile microscope assisted by AI for the detection of T. trichiura and A. lumbricoides in stool samples using microscopy as a reference (Dacal et al., Reference Dacal, Bermejo-Peláez, Lin, Álamo, Cuadrado, Martínez, Mousa, Postigo, Soto, Sukosd, Vladimirov, Mwandawiro, Gichuki, Williams, Muñoz, Kepha and Luengo-Oroz2021). The design of the digital mobile microscope consisted of a smartphone coupled to a conventional microscope (Leica DM-200) with the help of an adapter such that the smartphone's camera aligns with the optics of the microscope. For this study, 51 KK slides were prepared from 12 samples, imaged and uploaded into a telemedicine platform for remote image annotation, training and validation of AI algorithm. The sensitivity and specificity of their setup at image level were computed (see Table 3). According to the authors, the proposed design could serve a great deal to reduce time, distance and expertise needed for coproscopic analysis of STHs detection thereby making accurate diagnosis feasible. Nevertheless, this design still requires an expert to manually operate the attached microscope for sample imaging thereby sharing similar challenges as conventional microscopy.
The sixth proof-of-concept study by Oyibo et al. reported the SchistoScope; an automated microscope builds from off-the-shelf components for use in resource-limited settings for the detection of S. haematobium eggs with AI (Oyibo et al., Reference Oyibo, Jujjavarapu, Meulah, Agbana, Braakman, Van Diepen, Bengtson, Van Lieshout, Oyibo, Vdovine and Diehl2022). The prototype consists of an optical system with an automated 3-axis scanner (XYZ) to automate sample scanning process and an onboard computer connected to a Raspberry Pi HQ camera. Both urine samples from uninfected persons spiked with eggs from gut tissue of hamsters, clinical urine and stool samples were used to test the prototype. This tool captured quality images with distinguishable features of S. haematobium, S. mansoni and hookworm eggs. A dice similarity coefficient (DSQ) of 0.44 was obtained when evaluating the egg count outcome from the automated SchistoScope compared to manual counts. Based on the authors, the SchistoScope could be a suitable tool for use in the monitoring and evaluation of schistosomiasis control programmes in endemic settings. However, we think that the SchistoScope's performance in automated egg quantification based on the reported DSQ limits its potential application in monitoring and evaluation of schistosomiasis control programmes.
In 2 non-smartphone-based validation studies, a commercially available research-grade handheld mobile light microscope, Newton Nm1, was evaluated for the diagnosis of STH infections with conventional light microscopy as the reference standard (Bogoch et al., Reference Bogoch, Andrews, Speich, Ame, Ali, Stothard, Utzinger and Keiser2014a). In this study, 91 KK slides were randomly selected from a pack already analysed with conventional microscopy. These slides were re-examined using the Newton Nm1 microscope of which, 39.6 and 70.3% were positive for A. lumbricoides and T. trichiura, respectively, whereas 38.5% and 76.9% were positive for A. lumbricoides and T. trichiura, respectively, using conventional microscopy. No other known and highly sensitive reference test was used to determine whether samples were truly or falsely positives. Based on these findings, the authors concluded that the Newton Nm1 microscope may serve as a useful tool to detect and quantify STH eggs in endemic settings. Interestingly, Bogoch et al. (Reference Bogoch, Coulibaly, Andrews, Speich, Keiser, Stothard, N'goran and Utzinger2014b), and Coulibaly et al. (Reference Coulibaly, Ouattara, D'ambrosio, Fletcher, Keiser, Utzinger, N'goran, Andrews and Bogoch2016b), again evaluated the Newton Nm1 microscope for the detection of helminth eggs with conventional light microscopy (single KK and urine filtration) as a reference standard. From 164 prepared stool slides and 180 urine slides, Bogoch et al. determined the sensitivity and specificity of the device for the detection of Schistosoma and T. trichiura (Bogoch et al., Reference Bogoch, Coulibaly, Andrews, Speich, Keiser, Stothard, N'goran and Utzinger2014b). A sufficient diagnostic yield was obtained (see Table 3). Furthermore, Coulibaly et al. conducted a cross-sectional study and used urine and KK slides prepared from urine and stool samples collected from 226 individuals (Coulibaly et al., Reference Coulibaly, Ouattara, D'ambrosio, Fletcher, Keiser, Utzinger, N'goran, Andrews and Bogoch2016b). The Newton Nm1 handheld microscope produced a good sensitivity and an excellent specificity (see Table 3). The authors of these studies concluded that the Newton Nm1 microscope may serve as a valuable tool in clinical and public health settings to detect these infections. Nonetheless, both studies used conventional microscopy as the reference with limited sensitivity. Therefore, thorough evaluation with more sensitive diagnostics is needed to validate this tool. Furthermore, the Newton Nm1 microscope for the detection of these parasites is not fully automated.
The last study evaluated, for the first time, a new and remote diagnostic technique used in veterinary medicine called FECPAK compared to a single, double and quadruplicate KK method for analysing STHs eggs in human stool (Moser et al., Reference Moser, Barenbold, Mirams, Cools, Vlaminck, Ali, Ame, Hattendorf, Vounatsou, Levecke and Keiser2018). The FECPAK system is made up of a filtration unit, a micro-I cassette with sample loading wells that fit into the micro-I cassette, all connected to a computer for imaging of samples in a single FOV. For this study, 590 positive and 25 negative samples for any of the STHs were collected and analysed by the 4 methods. Images captured with the FECPAK were analysed by a microscopist. The sensitivity and specificity of the FECPAK device were determined for the detection of A. lumbricoides, T. trichiura and hookworm (Table 3). The sensitivity of FECPAK was found to be lower than single, double and quadruple KK method for the detection of A. lumbricoides and hookworm (see Table 3). The FECPAK underestimated the prevalence of these helminth infections compared to the single, double and quadruplicate KK method. Although FECPAK was originally developed for animal helminths diagnosis, authors concluded that it might be an interesting tool for both epidemiological and clinical human studies. Further evaluation studies of FECPAK for diagnosis of human STHs would help to further develop the tool.
Moving beyond test performance and readiness for LMIC
A systematic assessment of innovative optical diagnostic devices for parasitic infection diagnosis is important to understand the progress towards field implementation. In this review, we propose the use of a modified TRL scale as an appropriate standardized approach towards this goal. The FECPAK and the Newton Nm1 microscope met the criteria of the rank TRL-9 on the adapted TRL scale. In addition, of the 3 studies that validated the Nm1 microscope, only Coulibaly et al. evaluated the device for egg quantification which limits its application in MDA impact assessment programmes (Wiegand et al., Reference Wiegand, Secor, Fleming, French, King, Deol, Montgomery, Evans, Utzinger, Vounatsou and De Vlas2021). The Foldscope, although commercially available, was integrated with an iPhone S5 and validated in a field laboratory setting. This combined optical set-up is not yet in the market as an integrated diagnostic system, so we ranked it at TRL-4. Based on our scaling, 21% of the devices reviewed here are simply a demonstration of proof-of-concept (TRL-3), 55% in a stage of validation in the technical/field laboratory facility or community endemic setting (TRL-4, 5), 8% validated on clinical samples and the WHO recommended sensitivity and specificity met (TRL-6), and 15% are already commercialized (TRL-8, 9) (see Tables 2 and 3). Overall, validation in field settings (TRL-5, 6, 7) was revealed to be a bottle neck for 65% of these devices. While further research and development is needed to transition these devices to a commercial level, thorough validation before and after commercialization is required in field settings. Features beyond its performance, such as robustness, portability, technical simplicity and feasibility of repair and costs, are also crucial parameters to consider for readiness in LMICs.
Non-smartphone-based microscopes developed so far have different cost ranges which may depend on their designs and the functional parts used. The Newton Nm1 microscope, for example, has a price range from US$465 to US$930 depending on the series. The WHO TPP for STH and schistosome infection does not mention the minimum capital cost per tool required for control and elimination of these infections; however, a diagnostic test should cost less than US$3 per test. Aside from Armstrong et al., the reviewed articles did not report material cost analysis per test.
Conclusion and future perspective of optical devices with AI for soil-transmitted helminth and Schistosoma spp. detection
Simple, inexpensive, portable and robust diagnostic tools are urgently needed in resource-limited settings for the diagnosis of human helminthiasis using urine and stool samples. Important advancements have been made in the clinical sector with optical devices for automated malaria parasite detection (Frean, Reference Frean2008; Kaewkamnerd et al., Reference Kaewkamnerd, Uthaipibull, Intarapanich, Pannarut, Chaotheing and Tongsima2012; Coulibaly et al., Reference Coulibaly, N'goran, Keiser, Ouattara, Bogoch, Andrews and Bonfoh2016a; Hashimoto et al., Reference Hashimoto, Yatsushiro, Yamamura, Tanaka, Sakamoto, Ido, Kajimoto, Bando, Kido and Kataoka2017; Agbana et al., Reference Agbana, Diehl, Van Pul, Khan, Patlan, Verhaegen and Vdovin2018; Kumar et al., Reference Kumar, Verma, Shrivas, Thota, Singh, Rajasubramaniam, Das and Bharti2020; de Melo et al., Reference De Melo, Netto, Mwangi, Salazar, De Souza Sampaio, Monteiro, De Almeida e Val, Rocheleau, Thota and Lacerda2021). Comparatively, the automated optical detection of human stool and urine parasites such as STHs and schistosomes is still in its early stages. With advancing technology, optical devices could be developed to have a fully functioning computing system, with the potential to accommodate specific features such as imaging sensors, global positioning system (GPS) navigation, internet access, data storage and high processing power, each with added values for different stakeholders in the health system. This would facilitate data generation and distribution making it useful for mapping of STHs, Schistosoma spp. infections and impact assessment of MDA programmes.
Automation of such devices makes them comparatively easy to use and facilitates multitasking during sample analysis. In this review, we summarized 18 publications presenting 21 different innovative optical devices validated within the last decade for the diagnosis of soil-transmitted helminthiasis and schistosomiasis. Some of these devices require infrastructure for sample processing which poses a limitation to device application in resource-limited settings. Most device prototypes reported in this review require human expertise for image interpretation before conclusive decisions could be reached. Fully automated devices with integrated reliable AI models could potentially mitigate the chances of human error and reduce the need for human training. Currently, only a few studies have reported the use of optical devices with AI to detect STH and Schistosoma spp. infections in human stool and urine (Linder et al., Reference Linder, Grote, Varjo, Linder, Lebbad, Lundin, Diwan, Hannuksela and Lundin2013; Holmstrom et al., Reference Holmstrom, Linder, Ngasala, Martensson, Linder, Lundin, Moilanen, Suutala, Diwan and Lundin2017; Agbana et al., Reference Agbana, Nijman, Hoeber, Van Grootheest, Van Diepen, Van Lieshout, Diehl, Verhaegen, Vdovine and Coté2020; Dacal et al., Reference Dacal, Bermejo-Peláez, Lin, Álamo, Cuadrado, Martínez, Mousa, Postigo, Soto, Sukosd, Vladimirov, Mwandawiro, Gichuki, Williams, Muñoz, Kepha and Luengo-Oroz2021; Armstrong et al., Reference Armstrong, Harris, D'ambrosio, Coulibaly, Essien-Baidoo, Ephraim, Andrews, Bogoch and Fletcher2022; Oyibo et al., Reference Oyibo, Jujjavarapu, Meulah, Agbana, Braakman, Van Diepen, Bengtson, Van Lieshout, Oyibo, Vdovine and Diehl2022). Much less similar reports have been written for stool samples (Holmstrom et al., Reference Holmstrom, Linder, Ngasala, Martensson, Linder, Lundin, Moilanen, Suutala, Diwan and Lundin2017; Dacal et al., Reference Dacal, Bermejo-Peláez, Lin, Álamo, Cuadrado, Martínez, Mousa, Postigo, Soto, Sukosd, Vladimirov, Mwandawiro, Gichuki, Williams, Muñoz, Kepha and Luengo-Oroz2021). The focus on automated detection of parasites in urine rather than stool illustrates the challenges with stool sample analysis and re-affirms the need for more research and development exploring ways to overcome challenges of stool parasite detection with AI. In this regard, a study is currently ongoing with the aim to integrate automated algorithms into different low-cost innovative optical devices for the detection and quantification of STH and Schistosoma infections. The performance evaluation of the device is being evaluated in field settings in Nigeria and Gabon through the INSPiRED WP-3 (ClinicalTrials.gov identifier: NCT04505046) project (INSPiRED-project; http://inspired-diagnostics.info).
The final rank from the 19 different devices classified in this review reveals that 85% are not ready for commercialization (TRL<8). This is indicative of the need for more research and development on optical devices to detect STH and Schistosoma infections. Furthermore, stakeholder engagement is recommended (Levecke et al., Reference Levecke, Coffeng, Hanna, Pullan and Gass2021) as it provides further information on stakeholder desired products and context-specific needs that in turn accelerate its readiness and enable local productivity. In addition, before such devices become commercially available, validation of their diagnostic performance and operational characteristics by healthcare professionals and public health teams is required, in laboratory, clinical and field settings (Banoo et al., Reference Banoo, Bell, Bossuyt, Herring, Mabey, Poole, Smith, Sriram, Wongsrichanalai, Linke and O'brien2006). A proper diagnostic performance evaluation requires an appropriate and justifiable sample size to ensure a meaningful value of sensitivity and specificity with a sufficient level of power and low type-1 errors (Bujang and Adnan, Reference Bujang and Adnan2016). For all the diagnostic performance evaluation studies reviewed here, no justifiable sample sizes were used which decreases the power and increases the likelihood for type-1 error. Also, the choice of reference tests used to evaluate these diagnostic devices is crucial. In an ideal situation, a reference test should be 100% sensitive and specific which is usually not the case for any of the soil-transmitted helminthiasis and schistosomiasis tests reported. In such a case, a composite of 2 or more reference tests to match-up this specification is encouraged. All the studies reviewed here used conventional microscopy as a reference standard whose sensitivity may vary enormously based on the settings and experience of the microscopists. Thus, the performance of the diagnostic tool is likely to be underestimated. While evaluating such tools aiming for a certain diagnostic performance, the analysis time needed to develop a test result per device is crucial. Only Sukas et al. reported the time of analysis of the LOD (15 min).
Of all the parasites of interest from the studies included in this review, hookworm was the least represented. Only 2 out of the 18 studies reported on diagnosis of hookworm infection. We envisage that this might be due to the rapid disintegration of hookworm eggs in a KK smear, which hampers proper comparison between diagnostic procedures. Several diagnostic parameters were used to evaluate these optical devices depending on the goal for which it was developed. PPV and NPV, for example, which are important parameters for clinician's decision making about diagnosis, were not evaluated for most of the studies. This is because most of the optical devices were developed for epidemiology/research surveys and not for point of care. We conclude that there is still much to be done to get automated microscopes ready for application in areas with limited resources. Such settings will require reliable and simple novel diagnostics that are equally good or superior to conventional microscopy which also aligns with the WHO TPP.
Data availability
The authors confirm that the data supporting the findings of this review are available within the article.
Author's contribution
B. M. T, C. H. H, L. V. L., T. E. A. and A. A. A. conceived the review. B. M. T. searched the literature and drafted the manuscript. M. B. designed the figures. B. M. T. and T. E. A. developed the adapted scale; M. B., J.-C. D. and L. V. L. and reviewed it. M. B., L. V. L., A. A. A., A. K., J.-C. D. and T. E. A. revised and edited the manuscript. All authors edited and revised the final manuscript.
Financial support
This work was supported by the Netherland organization for scientific research NWO-WOTRO Science for Global Development programme, grant number W 07.30318.009 (INSPiRED – INclusive diagnoStics for Poverty REIated parasitic Diseases in Nigeria and Gabon).
Conflict of interest
None.
Ethical standards
Not applicable.