The use of atypical antipsychotics has increased dramatically since their introduction in the 1990s. Expenditure on atypical antipsychotics in the West Midlands rose by 105% between July 1999 and September 2002 and the number of prescriptions increased fivefold between 1996 and 2001 (Reference Ashcroft, Frischer and LockettAshcroft et al, 2002). Within the study period reported here (1994-2001), these drugs were solely licensed for the treatment of schizophrenia, with the exception of risperidone, which was licensed for ‘acute and chronic psychosis’. This diagnostic specificity is in contrast to the broader licensed indications for many conventional antipsychotics when initially licensed. In the UK, clozapine was licensed in 1989, risperidone in 1993 and olanzapine in 1996.
In June 2002, The National Institute for Clinical Excellence (NICE, 2002) recommended the first-line use of atypical antipsychotics for the treatment of schizophrenia along with further research in their use. Health organisations are expected to implement NICE technology assessments within 3 months. Funding is made available centrally for implementing NICE guidance, although incorporation in local budgets may not be transparent. More recently, funding is via generic uplifts, which further cloud the allocation of NICE budgets. In reality the National Health Service has struggled to keep up with the pace of output from NICE (Reference ShannonShannon, 2003).
In the UK, the Medicines Act 1968 was introduced to control licensing of medications, following difficulties with drugs such as thalidomide. The licensing process is administered by the Medicines Control Agency on behalf of health ministers who are advised by the Committee on Safety of Medicines. This was the sole licensing route for medicines in the UK prior to the establishment of the European Medicines Evaluation Agency (EMEA) in 1995. The EMEA was established to coordinate the processing of European Union licence applications in order to minimise duplication and ensure regulatory homogeneity throughout the Union. Currently, this process is under review, but since its establishment drug companies have usually chosen this route if they wish to market their product in more than one country of the European Union.
Once a medicine is licensed a marketing authorisation is granted and the clinical indications, dosage, precautions and other information derived from the marketing authorisation are presented as the summary of product characteristics. Pharmaceutical companies may only promote their product for the indications and uses given in this summary. For example, Pfizer, the world's largest drug manufacturer, was fined $392 m in 2004 for promoting gabapentin off-label (Reference LenzerLenzer, 2004). Patient information leaflets must reflect the information in the summary of product characteristics and are legally required to be issued with dispensed medication.
Using unlicensed medicines or using licensed medicines outside the parameters of the marketing authorisation (off-label) is not illegal or inappropriate and is accommodated in the Medicines Act 1968. However, if a doctor prescribes off-label then he or his employers have increased liability, as the pharmaceutical company would now be only liable for defects in the manufacturing process (Consumers’Association, 1992). However, refusing to prescribe off-label may also have legal implications, especially as off-label prescribing indications are often described in standard medical textbooks as the treatment of choice (Reference HenryHenry, 1999,Reference HenryHenry, 1999).
Method
We identified patients in secondary care that were prescribed atypical antipsychotics in North Staffordshire (population 460 000) between 1994 and 2001. Prescriptions were identified from hospital pharmacy records and confirmed by analysis of case notes. The ICD-10 diagnosis (World Health Organization, 1993) at time of prescription was obtained from case notes. Previous research in North Staffordshire has shown satisfactory diagnostic accuracy using this method (Reference Boardman, Hodgson and LewisBoardman et al, 1997). Only the index atypical antipsychotic prescribed was considered for patients treated with more than one atypical in the study period.
Results
A total of 502 patients were identified. Of these, 52.8% were male and the mean age was 41.8 years. Mean daily doses were: clozapine 332.3 mg (s.d.=150.3); olanzapine 12.1mg (s.d.=4.6); and risperidone 4.7 mg (s.d.=2.4). Two patients with schizophrenia were prescribed doses above those recommended in the British National Formulary (British Medical Association & Royal Pharmaceutical Society, 2002). Both were prescribed 30 mg of olanzapine.
Only one patient was prescribed clozapine off-licence (Table 1). Olanzapine was the most commonly prescribed atypical antipsychotic in North Staffordshire and was prescribed for schizophrenia in 176 of 310 prescriptions (56.8%). Risperidone was used on-label in 72 of 131 prescriptions (55%) and 63.4% of prescriptions were within licence, if the licence was interpreted as including affective psychosis.
Table 1. Prescription of atypical antipsychotic according to ICD-10 diagnosis
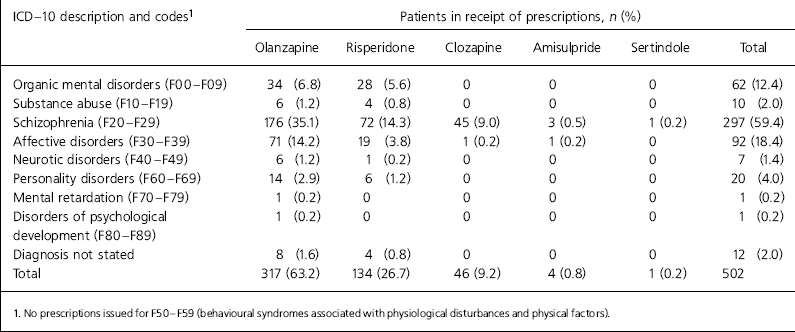
| ICD — 10 description and codes1 | Patients in receipt of prescriptions, n (%) | |||||
|---|---|---|---|---|---|---|
| Olanzapine | Risperidone | Clozapine | Amisulpride | Sertindole | Total | |
| Organic mental disorders (F00-F09) | 34 (6.8) | 28 (5.6) | 0 | 0 | 0 | 62 (12.4) |
| Substance abuse (F10-F19) | 6 (1.2) | 4 (0.8) | 0 | 0 | 0 | 10 (2.0) |
| Schizophrenia (F20-F29) | 176 (35.1) | 72 (14.3) | 45 (9.0) | 3 (0.5) | 1 (0.2) | 297 (59.4) |
| Affective disorders (F30-F39) | 71 (14.2) | 19 (3.8) | 1 (0.2) | 1 (0.2) | 0 | 92 (18.4) |
| Neurotic disorders (F40-F49) | 6 (1.2) | 1 (0.2) | 0 | 0 | 0 | 7 (1.4) |
| Personality disorders (F60-F69) | 14 (2.9) | 6 (1.2) | 0 | 0 | 0 | 20 (4.0) |
| Mental retardation (F70-F79) | 1 (0.2) | 0 | 0 | 0 | 0 | 1 (0.2) |
| Disorders of psychological development (F80-F89) | 1 (0.2) | 0 | 0 | 0 | 0 | 1 (0.2) |
| Diagnosis not stated | 8 (1.6) | 4 (0.8) | 0 | 0 | 0 | 12 (2.0) |
| Total | 317 (63.2) | 134 (26.7) | 46 (9.2) | 4 (0.8) | 1 (0.2) | 502 |
Discussion
Our results show high levels of off-label prescribing. Rates of off-label prescribing would have been even higher if we had restricted our coding to F20 of the ICD-10 by not including schizoaffective disorder, delusional disorder and transient psychotic disorders. Also, we did not assess contraindications or prescribing outside the licensed age range. Our results indicate that it cannot be assumed that the growth in atypical antipsychotic prescribing noted by Ashcroft et al (Reference Ashcroft, Frischer and Lockett2002) is for patients with schizophrenia. This would also suggest that atypical prescribing rates cannot be assumed to be an accurate reflection of adherence to NICE guidance for schizophrenia (NICE, 2002).
This prescribing may be clinically appropriate but it has a large impact on prescribing budgets. For example, prescribing costs for 1 year at the mean prescribed North Staffordshire dose are £1493 and £1094 for olanzapine and risperidone, respectively. Potentially, much of this funding may not be directly financed by NICE and this may cause dilemmas for clinicians, users and trusts. Clinicians may be discouraged from using diagnoses other than schizophrenia for patients with psychotic disorders, so that the most appropriate drug for clinical use can be funded.
Douglas-Hall et al (Reference Douglas-Hall, Fuller and Gill-Banham2001) reported similar off-label prescribing rates for atypical antipsychotics and, as in our study, that olanzapine was the only atypical antipsychotic prescribed above its licensed dose. Lowe-Ponsford & Baldwin (Reference Lowe-Ponsford and Baldwin2000) reported that 65% of psychiatrists acknowledged that they had prescribed off-label in the preceding month, although Douglas-Hall et al (Reference Douglas-Hall, Fuller and Gill-Banham2001) reported that only 10% of off-label prescribing was recorded in the patient's notes. Off-label prescribing is common in paediatrics, oncology and infectious diseases (Reference Conroy, Choonara and ImpiciatoreConroy et al, 2000; Reference McIntyre, Conroy and AveryMcIntyre et al, 2000).
Licensed indications for antidepressants have become more specific and the reasons for this have been debated (Healey, 2002). This trend to more indication-specific licensing is likely to continue owing to regulatory requirements. Pharmaceutical companies are unlikely to pursue licences for indications that are uneconomical. Also further studies to extend licensed indications may be counterproductive. This is exemplified in psychiatry by an apparent increase in the rate of cerebrovascular adverse events noted in trials of olanzapine and risperidone for behavioural problems in patients with dementia. The subsequent warning from the Committee on Safety of Medicines (2004) has implications for psychiatrists prescribing off-label in this patient group and necessitated a change in the summary of product characteristics for both these drugs. However, the growth in atypical antipsychotic prescribing for patients with dementia was partly related to concerns about the safety of typical antipsychotics, such as thioridazine.
Given delays in the licensing process, clinicians may anticipate changes in dosing strategies or use in other indications. For example, after our study was completed, olanzapine received a licence for ‘the treatment of moderate to severe manic episode’, and more recently, ‘in patients whose manic episode has responded to olanzapine treatment, olanzapine is indicated for the prevention of recurrence in patients with bipolar disorder’. These further indications would have reduced olanzapine off-label prescribing to 22%.
Declaration of interest
This research was funded by an unrestricted grant from Eli Lilly to Richard Hodgson.
Acknowledgements
We thank Professor Gilbert MacKenzie and Dr Yasin Al-tawarah for statistical advice.




eLetters
No eLetters have been published for this article.