Dietary guidelines worldwide recommend nutritious and health-promoting diets. In Australia, the Australian Dietary Guidelines encourage eating from the five core food groups: (i) fruit; (ii) vegetables and legumes/beans; (iii) lean meats and poultry, fish, eggs, tofu, nuts and seeds and legumes/beans; (iv) grain (cereal) foods; and (v) milk, yoghurt, cheese and/or alternatives( 1 ). Non-core foods and beverages are called ‘discretionary foods and beverages’ (DF). DF are defined as foods and drinks not necessary to provide the nutrients the body needs, and are high in saturated fat, added sugars, added salt or alcohol and low in fibre( 2 ). Other descriptive terms for DF include ‘energy-dense, nutrient-poor foods’, ‘empty calories’, ‘extra foods’ and ‘high-energy-dense foods’( Reference Albar, Alwan and Evans 3 – Reference Reedy and Krebs-Smith 6 ). The Australian Dietary Guidelines recommend DF to be consumed only occasionally and in small amounts, from 0 to 3 servings for males and 0 to 2·5 servings for females per day, with one serving containing 600 kJ( 7 ). The guidelines recommend that any additional energy requirements, such as for those who are taller or more physically active, should be met with servings from the core food groups or unsaturated spreads and oils in preference to discretionary choices( 1 ). Those who are overweight have no additional energy allowance and discretionary intake is discouraged, because the consumption of DF has been shown to increase the risk of weight gain and chronic disease( 1 , Reference Cohen, Sturm and Lara 8 – Reference Pereira, Kartashov and Ebbeling 11 ).
Sixty-three per cent of Australian adults are overweight or obese( 12 ) and chronic disease is the leading contributor to morbidity and mortality( 13 ). Therefore a large proportion of Australian adults have no energy allowance for DF, yet DF intake contributes 35 % of their total energy intake( 14 ). Large portion sizes may contribute to the overconsumption of DF. In an analysis of the 2011–12 National Nutrition and Physical Activity Survey (NNPAS) in Australia, the median portion size of cakes, savoury pastries, hamburgers and pizza exceeded the standard serving set by the dietary guidelines of 600 kJ, by up to 400 %( Reference Zheng, Wu and Louie 15 ). Given that the Australian Dietary Guidelines recommend no more than 3 DF servings daily for the majority of adults, many will be exceeding this limit from just a single portion of DF. At the same time, only 3·5 % of adult males and 5·2 % of adult females met the recommended daily servings of vegetables and legumes/beans, and 29·3 % of adult males and 23·0 % of adult females met the recommended daily servings of fruit( 16 ). With high discretionary intakes and low consumption of fruit and vegetables, DF could displace core foods, as reported in the USA( Reference Kant 4 ). Inadequate intake of core foods and excess consumption of discretionary foods may lead to the double burden of under- and overnutrition( Reference Piernas, Wang and Du 17 ). Hence, it is important to gain a deeper understanding of the role of DF in the diet of Australian adults to develop targeted strategies to assist in reducing its intake. While many studies focus on selected discretionary foods or beverages (e.g. sugar-sweetened beverages, salty snacks or foods high in saturated fats), a more thorough analysis of DF consumption patterns, including the highest energy contributors, in combination with consumer sociodemographic factors and adiposity-related measures, is needed to assist both health professionals and government in developing public health strategies to address the excess intake of DF.
The aim of the present study was to examine, in a nationally representative sample of the Australian adult population, the prevalence of DF consumption, the top DF food groups in terms of contribution to discretionary energy, their contribution to total sugars, saturated fat and Na intakes, and the eating occasions (EO) where the most DF were consumed. We analysed these nutrients because they form the basis of the definition of DF( 2 ) and we wanted to see what influence DF had on the nutrients that should be limited. At the time of analysis, data on added sugars and salt were not available so we chose total sugars as a substitute for added sugars and Na as a substitute for salt. Further, we aimed to examine the sociodemographic and lifestyle characteristics of DF consumers and any associations of DF with adiposity-related measures.
Methods
Survey methodology
The Australian Bureau of Statistics conducted the Australian Health Survey (AHS) between 2011 and 2013 on a nationally representative sample of Australians aged 2 years or over. The 2011–12 NNPAS was part of the AHS and collected physical activity data and detailed dietary information from 12 153 respondents. Trained interviewers used the automated multiple-pass method, which was developed by the Agricultural Research Service of the US Department of Agriculture( Reference Bliss 18 ) and adapted to reflect the Australian food supply, to capture all foods and beverages consumed by respondents within the 24 h prior to the interview day. A second day of dietary recall was provided by telephone interview by approximately two-thirds (n 7735) of participants. Total energy and nutrient intakes were derived from Food Standards Australia New Zealand’s customised nutrient composition database (AUSNUT)( 19 ). To maximise the sample size, data from the first 24 h recall among 9341 adults aged 19 years or over were utilised. A sensitivity analysis was performed to investigate whether there were differences in DF consumed between day 1 and day 2 of recall. The top foods that contributed to discretionary energy were similar across the two days (see online supplementary material, Supplementary Table 1). Further survey details are available from the Australian Bureau of Statistics’ website in the Australian Health Survey: Users’ Guide, 2011–13 ( 20 ).
Participants were classified by age group (19–30, 31–50, 51–70, ≥71 years), sex, socio-economic status (SES), physical activity level, adiposity-related measures, usual fruit and vegetable servings, and smoking status. The Socio-Economic Indexes for Areas (SEIFA)( 21 ) was used to define SES. SEIFA is a product developed by the Australian Bureau of Statistics that ranks areas in Australia into quintiles according to relative socio-economic advantage or disadvantage in terms of people’s access to material and social resources and their ability to participate in society. The lowest SES quintile was defined as the first quintile and the highest as the fifth quintile.
Physical activity was self-reported as the amount of physical activity each respondent undertook in the week prior to the survey. The interviewers collecting the information relied on participants’ recall, as no recording devices or other instruments were used to measure physical activity. The total number of minutes of physical activity was recorded, with one session equivalent to 30 min of moderate-intensity physical activity. The amount of time spent sitting or lying down for work, transport and leisure during the week prior to the survey was also self-reported by respondents. Respondents were classified based on the duration and number of sessions of physical activity into three categories: inactive, insufficiently active or sufficiently active for health( 20 ).
Interviewers measured the height, weight and waist circumference of consenting participants. A stadiometer was used to measure height, digital scales to measure weight, and a metal tape measure across the top of the belly button to measure waist circumference. Measured height and weight were used to calculate BMI (kg/m2) and respondents were categorised based on the WHO definitions( 22 ). Respondents were categorised according to their measured waist circumference and classified based on their waist size into the WHO categories for level of risk of metabolic complications: not at risk (<80 cm for females, <94 cm for males), increased risk (≥80 and <88 cm for females, ≥94 and <102 cm for males) or substantially increased risk (≥88 cm for females, ≥102 cm for males)( 23 ).
Respondents were asked to specify the usual number of fruit and vegetable servings consumed per day from the following options: ‘don’t eat fruit/vegetables’, ‘less than 1 serve, ‘1 serve, ‘2 serves’, ‘3 serves’, ‘4 serves’, ‘5 serves’ or ‘6 or more serves’. To determine mean number of servings we defined ‘don’t eat fruit/vegetables’ as 0, ‘less than 1 serve’ as 0·5 and ‘6 or more serves’ as 6.
Under-reporters
BMR is the amount of energy needed for an individual’s minimum set of body functions necessary for life over a defined time period. BMR is given in kilojoules per 24h and calculated using age, sex and weight (kilograms) as variables with no adjustment for activity levels. The ratio of energy intake (EI) to BMR (EI:BMR) was used to calculate under-reporters as participants with implausibly low intakes. Participants were classified as under-reporters or not under-reporters based on the Goldberg( Reference Goldberg, Black and Jebb 24 ) cut-off limit of 0·9 for EI:BMR, which is the lower 95 % confidence limit for a single day of data for a single individual, allowing for day-to-day variation in energy intakes and errors in calculation of EI:BMR.
Dietary intake
DF are defined by the Australian Dietary Guidelines as foods and beverages not necessary to provide the nutrients the body needs, and are high in saturated fats, added sugars, added salt or alcohol and low in fibre( 2 ). The Australian Bureau of Statistics categorised food groups in the survey as discretionary or non-discretionary based on these definitions and the supporting documents that underpin the Australian Dietary Guidelines. There are discretionary food and beverage items, such as diet soft drinks, that do not contain any energy. While these foods and beverages did not contribute to results relating to discretionary energy intake, they were included in all other relevant results such as prevalence of discretionary consumers. Foods were classified primarily at the minor food group level (5-digit); where it was not possible to determine if a food was discretionary at this level, the unique food code level (8-digit) was used along with the nutrient profiling cut-offs used in the Australian Dietary Guidelines modelling document( 25 ). Discretionary food and beverage groups in the present study are reported at the sub-major food group level (3-digit). There was a total of 132 sub-major food groups in the AUSNUT database, and sixty of these food groups contained a discretionary food or beverage. Henceforth, discretionary food groups (or ‘DF food group’) refers to the discretionary foods and beverages in the sub-major food group.
As part of the survey, respondents were asked to identify the name of their reported eating occasion (REO) as well as the time they began consuming each food or beverage. There were eleven REO available for selection: breakfast, morning tea, brunch, lunch, afternoon tea, dinner, supper, snack, beverage/drink, extended consumption or other( 20 ). As the choice of REO was subjective, respondents could report similar eating patterns in different ways. For example, a glass of wine with the evening meal could be reported by one respondent as ‘dinner’ and by another respondent as ‘beverage/drink’. We defined an EO as the consumption of one or more foods or beverages at the same time with the same REO.
Statistical analysis
The mean DF intake in servings (1 serving=600 kJ), the proportion of total energy intake from DF and total daily energy intake were calculated. To investigate frequency and timing of DF intake, we calculated the proportion of the EO that contained DF and the percentage contribution of each REO to total DF energy. For food groups, we determined the prevalence of consumers of each DF food group among DF consumers and the mean intake, in servings and in grams, among consumers of each DF food group. We calculated the percentage contribution of each food group to total DF energy, and to total sugars, saturated fat and Na intakes among DF consumers. Among the top four REO that contributed the most to daily DF energy, we determined the top five sub-major food groups, the percentage of total DF energy that it contributed at the REO and the mean kilojoules among consumers of the food group. Quartiles of the percentage energy contribution from DF were used to classify adults as low consumers (quartile 1) and high consumers (quartile 4).
The statistical software package IBM SPSS Statistics version 23.0 was used for all analyses. Due to the large sample size and the number of tests, we felt that to classify P values <0·05 as significant would lead to many type 1 errors; so we treated P values <0·001 as significant. Data were weighted to the Australian population using weights provided by the Australian Bureau of Statistics. Descriptive summaries were calculated for all variables of interest. ANOVA tables were produced to calculate se of the mean. Associations between quartiles of DF energy contribution and demographic and adiposity-related measures and lifestyle characteristics were examined using Pearson’s χ 2 tests. General linear models were created using univariate ANOVA to estimate the effect of age group, sex, their interaction and quartiles of DF energy contribution on dietary metrics, adiposity-related measures and lifestyle characteristics. Post hoc pairwise comparisons using the Bonferroni correction were performed to show pairwise significance between quartiles of DF energy contribution.
Results
Almost all adults consumed DF (97·5 %; Table 1). Over 60 % of adults had more than the maximum recommended 3 DF servings/d, with a per capita mean of 5·0 (se 0·0). More than 50 % of males and females of all age groups, apart from females 51 years of age or over, consumed more than 3 DF servings/d. Young males and females aged 19–30 years had the highest daily mean DF servings (6·7 (se 0·2) servings/d for males, 4·6 (se 0·1) servings/d for females). Consumption of DF decreased with age for both males and females, with the ≥71 years age group having the lowest mean servings for each sex (4·9 (se 0·2) for males, 3·5 (se 0·1) for females). Males had a greater DF energy intake than females for all age groups, both in total and as a proportion of total energy intake. The proportion of total energy from DF was greater than 30 % for all age and sex groups except females aged 51–70 years (28·6 (se 0·5) %), and this age group also had the highest prevalence of non-consumers of DF (3·8 %). For each age group, males had a lower number of total daily EO than females, but more of their EO contained DF, and therefore the proportion of EO that contained DF was higher among males than females. About half of all EO contained DF: between 55 and 58 % for males and between 46 and 51 % for females.
Table 1 Baseline characteristics of the sample of Australian adults aged ≥19 years (n 9341) from the 2011–12 National Nutrition and Physical Activity Survey

DF, discretionary foods and beverages; EO, eating occasion.
* Includes those who consumed DF that do not contain energy, such as diet or zero-calorie soft drinks.
Which food groups contributed the most to discretionary intake?
Among DF consumers, the top four DF food groups ranked by contribution to daily DF energy intake were: cakes, muffins, scones, cake-type desserts (8·4 %); wines (8·1 %); pastries (8·0 %); and beers (6·1 %; Table 2). These top four food groups had large portion sizes, ranging from 2·3 to 3·0 DF servings; however, none of these food groups made the top four when ranked by popularity (prevalence of consumers). The top contributor to DF energy intake – cakes, muffins, scones, cake-type desserts – was consumed by only 16 % of DF consumers, but had a large mean portion size of 3 DF servings, or 123 g, per consumer. The top four food groups ranked by popularity (sugar, honey and syrups; soft drinks and flavoured mineral waters; processed meat; sweet biscuits) had between 22·4 and 48·5 % of consumers, and small mean portion sizes ranging between 0·5 and 1·1 mean DF servings per consumer. Sugar, honey and syrups was the most popular food group with 48·5 % of DF consumers, but the mean DF servings per consumer was 0·5, and it was ranked only seventh by contribution to total DF energy intake.
Table 2 Proportion of consumers, energy and nutrient contributions, and serving size of the top twenty sub-major food groups that contained discretionary foods and beverages (DF), ranked by percentage energy contribution, among Australian adults aged ≥19 years (n 9341) from the 2011–12 National Nutrition and Physical Activity Survey

Three of the top five DF food groups by contribution to DF energy intake were beverages: two alcoholic beverages (wines; beers) and one non-alcoholic beverage (soft drinks and flavoured mineral waters). Of the top twenty DF food groups, processed meat contributed the most to Na, pastries the most to saturated fat, and soft drinks and flavoured mineral waters the most to total sugars.
Which food groups, during which reported eating occasions, contributed the most to discretionary intake?
The top four REO by contribution to total discretionary energy were dinner, lunch, snack and beverage/drink, which in total contributed 75 % of total DF energy (Table 3). Lunch and dinner together contributed almost half (45 %) of all DF energy, and snack and beverage/drink almost a third (30 %). The top contributors at dinner, lunch, snack and beverage/drink were wines (15 %), pastries (17 %), chocolate and chocolate-based confectionery (21 %) and beers (25 %), respectively.
Table 3 Top five reported eating occasions (REO)Footnote * that contributed the most to total energy intake from discretionary foods and beverages (DF) and the top five sub-major food groups by percentage contribution to DF energy at each REO among Australian adults aged ≥19 years (n 9341) from the 2011–12 National Nutrition and Physical Activity Survey

* The remaining 25·3 % of DF consumption came from breakfast 8·2 %, brunch 0·4 %, morning tea 4·4 %, afternoon tea 4·7 %, supper 2·7 %, extended consumption 4·6 %, and other 0·3 %.
In addition to beers, the second-highest contributor to the REO beverage/drink was also an alcoholic beverage (wines contributed 20 %), and both these beverages were consumed in substantially higher portion sizes (at least three times more kilojoules per consumer) than the other food groups in this REO. The top four food groups that contributed to DF energy during snack were all significant sources of total sugars and saturated fat, whereas those at lunch and dinner were significant sources of Na and saturated fat. As well as being the top contributor to DF energy at lunch, pastries (croissants, Danish pastries, pies, sausage rolls, quiches, spring rolls, etc.) contributed the second-most DF energy to dinner. Other food groups that were top-five DF contributors in two different REO were soft drinks and flavoured mineral waters (lunch and beverage/drink) and cakes, muffins, scones, cake-type desserts (lunch and snack).
Characteristics of adults by proportion of energy from discretionary foods and beverages
The DF contribution to total daily energy in quartile 1 (low consumers) ranged between 0 and 16 % of energy and between 47 and 100 % for quartile 4 (high consumers; Table 4). There were differences in sociodemographic, dietary and lifestyle factors between high and low consumers. More than half of low consumers were female (55·6 %) and the prevalence of females decreased with increasing quartile. DF intake decreased with age: 59·2 % of low consumers were aged 19–50 years, compared with 65·2 % of high consumers. High consumers had a higher prevalence of adults from the lowest SES quintile compared with all other quartiles. There was a higher prevalence of low consumers who met physical activity guidelines based on duration and session: 46·5 % of low consumers met guidelines, and this decreased with increasing quartile, of which 38·3 % of high consumers met guidelines.
Table 4 Associations between quartiles of percentage energy contribution from discretionary foods and beverages (DF) and demographic, adiposity-related measures and lifestyle characteristics among Australian adults aged ≥19 years (n 9341) from the 2011–12 National Nutrition and Physical Activity Survey
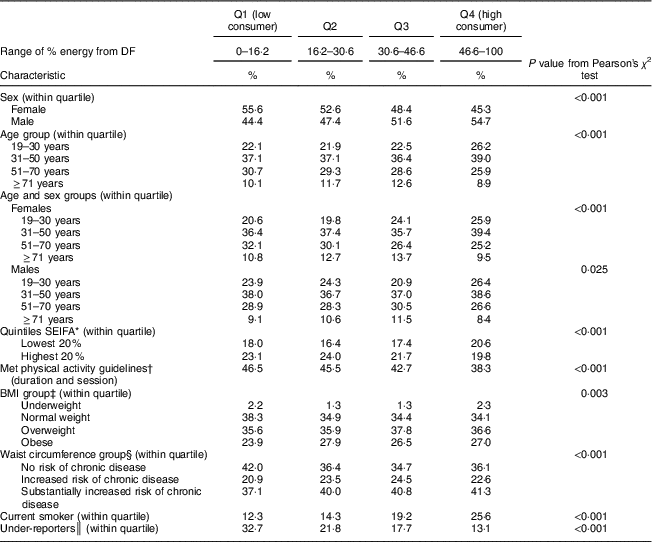
Q, quartile; SEIFA, Socio-Economic Indexes for Areas.
* SEIFA was developed by the Australian Bureau of Statistics and ranks areas in Australia according to their relative socio-economic advantage( 21 ).
† For adults aged ≥18 years, at least 150 min of physical activity over five or more sessions per week is recommended( 20 ).
‡ Based on BMI: underweight (<18·5 kg/m2); normal weight (≥18·5–<25·0 kg/m2); overweight (≥25·0–<30·0 kg/m2); obese (≥30·0 kg/m2)( 22 ).
§ Based on WHO cut-offs for waist circumference: not at risk of metabolic complications (<80 cm for females, <94 cm for males); increased risk of metabolic complications (≥80 and <88 cm for females, ≥94 and <102 cm for males); substantially increased risk of metabolic complications (≥88 cm for females, ≥102 cm for males)( 23 .
║ Participants were classified as under-reporters based on the Goldberg cut-off limit of an energy intake to BMR ratio of 0·9( Reference Goldberg, Black and Jebb 24 ).
The prevalence of overweight and obese adults was not significantly different across quartiles of DF intake. Prevalence of no risk of chronic disease was significantly higher among low consumers (42·0 %) than among high consumers (36·1 %).
The higher the quartile of DF energy, the higher the prevalence of smokers: 26 % of high consumers were smokers compared with 12 % of low consumers. Under-reporting of energy intake was more prevalent among low consumers: 33 %, compared with 13 % among high consumers.
Based on general linear models adjusted for age, sex and their interaction, total daily energy and DF energy intake both increased with increasing quartile (P<0·001), and high DF consumers had an average intake of 10·1 DF servings/d (Table 5). The number of daily EO was significantly lower among low consumers compared with the second and third quartiles (P<0·001) but not among high consumers. The number of EO with DF increased across quartiles: high consumers had more than double the EO with DF compared with low consumers (P<0·001). Similarly, the proportion of EO that contained DF doubled from 34·4 (se 0·4) % among low consumers to 67·6 (se 0·4) % among high consumers.
Table 5 Estimated effects of quartiles of percentage energy contribution from discretionary foods and beverages (DF) on diet, lifestyle and adiposity-related characteristics among Australian adults aged ≥19 years (n 9341) from the 2011–12 National Nutrition and Physical Activity Survey

Q, quartile; EO, eating occasion.
a,b,c,dMean values within a row with unlike superscript letters were significantly different (P<0·001 post hoc, Bonferroni).
* Adjusted for quartile of percentage energy from DF, age group, sex and their interaction using univariate ANOVA.
† Univariate ANOVA denote the effect of quartiles of percentage energy from DF.
‡ Age group, sex and their interaction were all significant (P<0·001, univariate ANOVA).
§ No variables (apart from quartile percentage of energy from DF) were significant in the model (univariate ANOVA).
║ Age group and sex were significant (P<0·001) but not their interaction (univariate ANOVA).
Low consumers had a higher number of minutes doing physical activity (236 (se 6) min) than high consumers (189 (se 6) min) and spent less time sitting or lying down for work, transport and leisure (35 h 25 min (se 27 min) v. 38 h 20 min (se 29 min); P<0·001).
There was no significant difference in mean BMI; however, low consumers had a significantly lower waist circumference (91·6 (se 0·3) cm) than adults in quartile 3 (93·4 (se 0·3) cm) and high consumers (93·9 (se 0·3) cm; P<0·001).
Self-reported usual daily fruit servings decreased with increasing DF quartile, and high DF consumers self-reported fewer daily vegetable servings (2·2 (se 0·0)) than low consumers (2·4 (se 0·0)) and those from the second quartile (2·5 (se 0·0); P<0·001).
The top five food groups by DF energy contribution among low consumers were those consumed in smaller portion sizes such as sugar, honey and syrups and sweet biscuits (Table 6). In contrast, the top DF food groups among high consumers were those consumed in larger portion sizes, such as pastries, cakes and alcoholic beverages.
Table 6 Top five sub-major food groups by quartiles of percentage energy contribution from discretionary foods and beverages among Australian adults aged ≥19 years (n 9341) from the 2011–12 National Nutrition and Physical Activity Survey

Q, quartile.
Results based on the same models are reported by age group and sex in the online supplementary material, Supplemental Table 2.
Discussion
The results of the present study demonstrate that virtually all Australian adults consumed DF, with an average intake of five DF servings; well above the maximum recommended three servings stipulated in the Australian Dietary Guidelines( 7 ). DF intake varied by sex and age, with younger males having the highest intakes. Food groups that contributed the most to DF energy intake (cakes, muffins, scones, cake-type desserts; wines; pastries; beers) were characterised by large portion sizes. Lunch and dinner combined contributed almost half of daily DF energy intake, with pastries and wines, respectively, the most popular at those occasions. High DF consumption was correlated with lower SES, physical activity, usual fruit intake and higher waist circumference, but not higher BMI.
The high intake of DF by Australian adults is consistent with other national nutrition surveys globally. A high intake of DF as a proportion of total energy intake has been reported in studies in Belgium, the USA, Mexico and Canada. In Belgium, 92 % of people aged 15 years or older consumed more than the maximum recommendation of less than 100 g of nutrient-poor, energy-dense foods daily( Reference Vandevijvere, De Vriese and Huybrechts 26 ). In the USA, energy-dense, nutrient-poor foods contributed 27 % of total energy intake and alcohol contributed an additional 4 %( Reference Kant 4 ). In Mexico, results from the national nutrition survey for people aged 5 years or older reported that sugar-sweetened beverages and foods high in saturated fat and/or added sugars contributed 26 % of total energy intake( Reference Aburto, Pedraza and Sanchez-Pimienta 27 ); and in Canada, non-core foods contributed 22·7 % of total daily energy for adults( Reference Garriguet 28 ). Direct comparisons between countries are limited due the lack of a universal definition for DF and different methodologies in food classification( Reference Auestad, Hurley and Fulgoni 29 ). In Australia, an analysis of the 1995 National Nutrition Survey reported that 99 % of adults consumed ‘extra’ foods and that these contributed 36 % of total energy intake( Reference Rangan, Schindeler and Hector 5 ). These findings suggest Australian DF intakes have largely remained unchanged since 1995, although there are minor differences in the definitions between ‘extra foods’ and ‘discretionary foods’ between the two surveys.
We found that young males were the highest consumers of DF, with more than half consuming five or more servings of DF in a day, similar to the previous 1995 National Nutrition Survey in Australia, where 19–24-year-old males had the highest percentage energy intake from ‘extra’ foods of all adults( Reference Rangan, Schindeler and Hector 5 ). Similar findings have also been reported globally. In the USA, 19–30-year-old males were the most likely to exceed the daily maximum DF allowances( Reference Krebs-Smith, Guenther and Subar 30 ); and in Canada, 19–30-year-old males, of all adult groups, consumed the greatest percentage of energy from ‘other foods’ outside the four main food groups( Reference Garriguet 28 ). Compared with females, males are less likely to have a high level of nutrition knowledge( Reference Worsley, Wang and Byrne 31 ), are less likely to be health conscious( Reference Fagerli and Wandel 32 , Reference von Bothmer and Fridlund 33 ) and are less likely to be involved in meal preparation( Reference Smith, McNaughton and Gall 34 ). Since eating behaviour can persist into later life( Reference Larson, Neumark-Sztainer and Story 35 – Reference Pedersen, Holstein and Flachs 37 ), there is a need for the development of DF-reducing interventions that target younger males.
Our analysis found that the top food groups that contributed to DF energy intake each had high mean DF servings per consumer, at quantities close or equal to three DF servings, the maximum recommended for an entire day (7 ). Interestingly, the most popular DF food groups – including sugar, honey and syrups; sweet biscuits; and soft drinks and flavoured mineral waters – were not among the highest contributors to DF energy intake as they had much smaller portion sizes. These smaller portion-sized food groups were more likely to be the top DF energy contributors among low DF consumers. However, the top food groups among high consumers were foods typically consumed in larger portion sizes. It is likely that portion sizes are an important contributing factor to high DF intake. There is considerable evidence that the portion sizes of many foods, particularly DF, have increased in the USA( Reference Nielsen and Popkin 38 , Reference Young and Nestle 39 ), the Netherlands( Reference Steenhuis, Leeuwis and Vermeer 40 ) and Australia( Reference Zheng, Wu and Louie 15 ), and it is well established that increasing portion size increases energy intake in well-controlled laboratory studies( Reference Ello-Martin, Ledikwe and Rolls 41 – Reference Kral, Roe and Rolls 44 ). Beverages may be of particular concern among high DF consumers. Three of the leading five food group contributors to DF energy intake among adults in the third and fourth quartiles of DF intake were beverages, compared with low DF consumers, who had no beverages in the leading five DF contributors. It has been demonstrated that beverages have a relatively low satiety value in comparison to solid foods( Reference Cassady, Considine and Mattes 45 – Reference Mattes and Campbell 47 ). Experimental evidence has shown that a reduction in portion size leads to a reduction in energy intake( Reference Stroebele, Ogden and Hill 48 ), including for discretionary foods( Reference Grieger, Wycherley and Johnson 49 ), even when there is no change to the total quantity of food offered( Reference Marchiori, Waroquier and Klein 50 ). Strategies to reduce DF consumption should therefore consider interventions that reduce portion size, such as the use of visual cues, labelling, reducing the size of packaging and plate size( Reference Benton 51 ). Selecting discretionary foods that are generally consumed in smaller portions, such as sugar, honey and syrups in coffee or tea, or processed meat in a sandwich, instead of those consumed in larger portions, such as cakes, muffins, scones, cake-type desserts or pastries, may also help to reduce total DF energy intake, since it may be more difficult to have smaller portions of the latter foods, such as a quarter of a piece of cake or half a muffin. Given that there is high variability in the manufacturer-declared serving sizes for DF in Australia( Reference Haskelberg, Neal and Dunford 52 ), and that inaccurate estimation of standard dietary servings and portion sizes may also contribute to excess consumption( Reference Abramovitch, Reddigan and Hamadeh 53 ), reference guidelines or regulatory policy concerning the development and labelling of serving sizes for DF should also be considered.
We found that dinner, lunch, snack and beverage/drink were the four leading EO that contributed to DF energy intake, with dinner and lunch combined contributing almost half of daily DF energy intake. Few studies have previously investigated the contribution of EO to DF energy intake( Reference Leech, Worsley and Timperio 54 ). In Mexico, the proportion of energy that products high in saturated fat and/or high in added sugar contributed was higher at mid-afternoon snacks and lower at lunch and brunch, while the proportion of energy from sugar-sweetened beverages was higher during mid-morning snacks( Reference Batis, Rodriguez-Ramirez and Ariza 55 ). In Australia, an analysis of the 2011–12 NNPAS reported that a higher frequency of snacks (self-reported by participants), but not main meals, was associated with a lower compliance to the Dietary Guidelines Index for discretionary foods( Reference Leech, Livingstone and Worsley 56 ). In our analysis, the REO snack contributed a high percentage of DF energy, but morning tea, afternoon tea and supper, which traditionally are also snacking occasions, did not. With more than half of all EO containing DF, encouraging adults to have EO free of DF may help to reduce its consumption.
Targeting high-DF EO with the provision of core foods may also be an effective means for reducing DF intake at these meal times, since the substitution of discretionary with core foods has been shown to reduce energy intake( Reference Grieger, Wycherley and Johnson 49 ). In a crossover trial that incorporated vegetables in place of other ingredients within a recipe to lower the energy density of the entire meal, intake of vegetables increased and reduced the total energy intake over the day, without changes to satiety( Reference Blatt, Roe and Rolls 57 ). In a national nutrition campaign in the UK, the promotion of ‘smart swaps’, or the replacement of discretionary foods with core foods, was associated with a change in purchase habits towards healthier products( Reference Wrieden and Levy 58 ).
Wines was the top food group at dinner and beers the top at beverage/drink that contributed to DF energy intake. The high energy contribution from alcohol is consistent with data that show alcohol consumption in Australia is among the highest in the world( 59 ). Alcohol is a top source of discretionary energy and, unlike other discretionary foods, can be an addictive drug( 60 ); since it is not necessary to meet nutritional needs, its reduction should be a top public health priority. The high contribution of alcoholic beverages to total DF energy intake reinforces the need for DF-reducing strategies to specifically target a reduction in alcohol intake.
We found that foods and beverages that were top sources of discretionary energy were not necessarily the top sources of the nutrients total sugars, saturated fat and Na. For example: wines, pastries and beers were among the leading contributors to DF energy but not to total sugars intake; and sugar, honey and syrups was among the leading contributors to total sugars intake but not DF energy intake. It should be noted, however, that these outcomes may have differed if added sugars had been analysed instead of total sugars although, by definition, total sugars from discretionary foods are mostly added. These findings suggest that it is a combination of portion size and the type of food that influences discretionary energy intake, and hence a focus on individual nutrients alone is unlikely to target the leading contributors to total DF energy intake in the diet of Australian adults. Our data strengthen the case for interventions aimed at reducing DF consumption that focus on targeted EO and whole diets rather than on singular nutrients of concern.
Compared with low consumers, high consumers were more likely to report lower physical activity, be a current smoker and have a lower usual fruit intake. The clustering of high DF intake with other less healthy lifestyle behaviours, such as lower physical activity and greater fast-food intake, has been previously reported in the USA( Reference Pereira, Kartashov and Ebbeling 11 ). Data from the National Health and Nutrition Examination Survey (NHANES) also reported that a high intake of energy-dense, nutrient-poor foods was associated with a low intake of core foods( Reference Kant 4 ). Interventions that appreciate these interrelationships may be more effective than interventions aimed at reducing DF alone.
Consistent with our finding, there is a large body of evidence that reports an association between lower SES and poorer diet quality( Reference Darmon and Drewnowski 61 ), including in Australia( Reference Grech, Sui and Siu 62 ), but these findings are not uniform across countries. High DF intake has been associated with a higher SES in Mexico( Reference Aburto, Pedraza and Sanchez-Pimienta 27 ) and a lower DF intake has been associated with lower income earners in the USA( Reference Kant 4 ). Since the relationship between diet quality and SES may differ according to the culture and economic status of the country, it is important to have population-specific data to best inform local intervention policies aimed at reducing DF intake. In Australia females of low SES reported that the perceived high cost of healthy food and a lack of time were important barriers for healthy eating( Reference Inglis, Ball and Crawford 63 ). Yet, it has been shown that healthy diets can be more affordable than current (less healthy) diets in Australia( Reference Lee, Kane and Ramsey 64 ) and that an education-based intervention on food affordability improves perceptions of healthy food affordability in Australian mothers( Reference Williams, Abbott and Thornton 65 ). Reducing the perception of the high cost and time commitments associated with healthy eating may be particularly important for reducing DF intake.
With respect to adiposity-related measures, we found a clear and consistent relationship with DF consumption and waist circumference, but not BMI. There is a large body of evidence that waist circumference independent of BMI is a significant predictor of chronic diseases( Reference Elbassuoni 66 – Reference Pischon, Boeing and Hoffmann 69 ). An association between DF or its components and waist circumference, but not BMI, has been reported previously. An analysis of the 1988–1994 NHANES reported that high waist circumference but not BMI was associated with the amount of daily energy intake from energy-dense, nutrient-poor foods in men( Reference Kant 4 ). An increase in waist circumference but not BMI was also associated with increasing frequency of sugar-sweetened beverage intake in a study of US adults( Reference Odegaard, Choh and Czerwinski 70 ). A number of prospective studies that measured increases in waist circumference adjusted for BMI have also reported an association with poorer diet quality( Reference Halkjaer, Sorensen and Tjonneland 71 – Reference Romaguera, Norat and Mouw 74 ). Increased waist circumference without changes to BMI may be a marker of increased visceral fat, and it has been suggested that some aspects of poor diet quality, such as high glycaemic index, may causally encourage greater visceral fat storage( Reference Romaguera, Angquist and Du 72 , Reference Du, van der and van Bakel 75 ). It is also possible that our observed associations are due to confounding factors or under-reporting. In our study, low DF consumers were more likely to meet recommendations for physical activity, for example, which generally reduces abdominal fat( Reference Kay and Fiatarone Singh 76 ). We also found a substantially higher proportion of under-reporting among the lowest consumers of DF (33 %) compared with the highest consumers of DF (13 %). This may be explained in part by a proportion of low DF consumers actively dieting and purposely reducing total energy and DF intake, and not under-reporting as predicted by EI:BMR. Overweight and obese individuals are more likely to under-report than lean individuals( Reference Macdiarmid and Blundell 77 ) and DF are more likely to be under-reported than other foods( Reference Lafay, Basdevant and Charles 78 , Reference Lafay, Mennen and Basdevant 79 ). Intervention studies are required to better understand the possible causal effects between different DF and the different measures of adiposity.
A particular strength of the present study is the use of a large, nationally representative sample of the Australian adult population. The data are cross-sectional and causal effects cannot be determined from the observed associations. Dietary intake was derived from a single day of 24 h recall and is not indicative of usual intakes. Two-thirds of respondents in the NNPAS provided two days of recall; however, in order to maximise the sample size only day 1 of recall was used. Evidence of under-reporting of energy intake in the survey implies that total DF intake could be even higher than reported. Since under-reporting is generally higher in females and overweight and obese respondents( Reference Macdiarmid and Blundell 77 ), under-reporting may have also impacted the observed associations between DF intake and other variables, such as sex, waist circumference and BMI.
Conclusions
In summary, the current results showed intakes of DF well above the maximum recommended levels stipulated by the Australian Dietary Guidelines, with the average per capita intake equivalent to five DF servings daily. The leading contributors to total DF energy intake were generally DF food groups consumed in large portion sizes, close or equal to three DF servings, the maximum DF energy limit recommended for an entire day. Dinner and lunch were the leading contributors to DF energy intake of all EO and contributed almost half of all DF energy intake, followed by the REO snack and beverage/drink. Addressing high DF intake by targeting the DF consumed in large portion sizes, particularly at dinner and lunch, may help to inform more strategic and evidenced-based DF-reducing interventions, for which the need is compelling.
Acknowledgements
Acknowledgements: The authors would like to thank the Australian Bureau of Statistics for conducting the survey. Financial support: Funding for this research was provided to Nutrition Research Australia by Nestlé Australia. J.K., an employee of Nestlé Australia, assisted with editing of the manuscript. The funding sponsors had no role in the design of the study; in the analyses or interpretation of data; or in the decision to publish the results. Conflict of interest: J.K. is an employee of Nestlé Australia, which provided study funding. All other authors declared no conflict of interest. Authorship: F.F.-M developed the research plan and had primary responsibility for the analysis and final content. A.M. and P.P were responsible for the data and statistical analyses. All authors assisted with the manuscript writing and have read and approved the final manuscript. Ethics of human subject participation: Not applicable as the study was a secondary analysis of existing national survey data. The Australian Bureau of Statistics collected the data under the Census and Statistics Act 1905.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980018003361








