Patients with elevated total cholesterol (TC) and LDL cholesterol (LDL-C) levels are at high risk of developing atherosclerosis and CHD(Reference Gotto1). First-line treatment normally focuses on lowering LDL-C, often accomplished by the use of statins. However, growing evidence suggests that non-HDL cholesterol (non-HDL-C) is a stronger predictor of CHD death than LDL-C(Reference Al-Daghri, Al-Attas and Al-Rubeaan2–Reference Packard and Saito7). Apart from statins, changes in lifestyle factors, such as quitting smoking(Reference Chelland Campbell, Moffatt and Stamford8), becoming more physically active and eating a healthy diet, can also influence LDL-C and non-HDL-C. In the last decade there has been more interest in changing dietary habits, with the appearance of functional foods. Since 1999, margarines containing phytosterols/phytostanols (phytosterols/-stanols) have become available on the US and EU market(Reference Fransen, de Jong and Wolfs9). Phytosterols/-stanols, which are structurally related to cholesterol, are thought to compete with cholesterol for solubilisation into mixed micelles. This leads to a reduced absorption of cholesterol and/or to an enhanced efflux of cholesterol back into the intestinal lumen due to a higher expression of the ABC transporter. Both mechanisms ultimately result in an increased faecal output of cholesterol(Reference de Jong, Plat and Mensink10–Reference Trautwein14).
Randomized controlled trials (RCT) have shown the efficacy (extent to which an intervention produces a beneficial effect under ideal conditions) of phytosterols/-stanols in lowering serum cholesterol levels: it is estimated that phytosterols/-stanols reduce TC and LDL-C by roughly 6 % and 10 %, respectively(Reference Eussen, Klungel and Garssen15–Reference Katan, Grundy and Jones17). It has been shown that phytosterols/-stanols are equally effective when used alone as part of the diet or when used as an adjuvant to ongoing statin therapy. Adding phytosterols/-stanols to statin therapy appears to be more effective than doubling the statin dose and, therefore, these products might especially be beneficial for persons who do not reach LDL-C goals with statin monotherapy and for those who experience side-effects from high doses of statins.
Although RCT are widely accepted as the gold standard of medical intervention research, their design may include short-term interventions, frequent follow-up visits, extensive monitoring and the use of restricted patient populations with high adherence to therapy. These factors limit extrapolation to daily practice populations(Reference Freemantle and Hessel18, Reference Silverman19). Because of the high adherence to therapy in RCT and the fact that poor adherence is thought to contribute to the failure of patients to achieve therapy targets(Reference Cramer, Roy and Burrell20, Reference Liberopoulos, Florentin and Mikhailidis21), the reductions of 6 % in TC as found in RCT may not be accomplished in persons who use the enriched margarines and statins under customary conditions.
The aim of the present study was to assess the effectiveness (extent to which an intervention works in daily medical practice) of the use of phytosterol/-stanol-enriched margarine in subjects using or not using statins in a real-world setting. As there are currently no standard databases available that integrate food intake and drug monitoring, data from an ongoing free-living cohort study containing information on functional food use was linked to a pharmacy-dispensing database for the purpose of the present study.
Subjects and methods
Study setting
Subjects from the Dutch Doetinchem Cohort Study and the Pharmacomorbidity-Record Linkage System (PHARMO-RLS) were linked using information on gender, date of birth and postcode in order to obtain information on the use of phytosterol/-stanol-enriched margarines and statins of the same subjects.
The Doetinchem Cohort Study was approved according to the guidelines of the Helsinki Declaration by the external Medical Ethics Committee of the Dutch TNO Research Institute. Linkage has been performed only for those participants who have agreed to it in their informed consent. The main objective of this ongoing cohort study is to investigate changes in lifestyle and risk factors for chronic diseases within patients over time in consecutive 5-year intervals. Details of the overall cohort study have been described elsewhere(Reference Verschuren, Blokstra and Picavet22). Participants who were examined between 1998 and 2002 and were re-examined at 5-year follow-up between 2003 and 2007 were included in the present analysis. On both examination days, respondents completed a general questionnaire and a validated FFQ(Reference Ocke, Bueno-de-Mesquita and Goddijn23, Reference Ocke, Bueno-de-Mesquita and Pols24). The general questionnaire contained questions on demographic and lifestyle factors. The 178-item semi-quantitative FFQ assessed habitual dietary intake. Daily energy and nutrient intakes were computed using an adapted version of the 1996 computerized Dutch food composition table(25). In addition, non-fasting blood samples were obtained on each examination day.
PHARMO-RLS comprises a database in which pharmacy-dispensing data are collected of a representative sample of more than 200 community pharmacies in fifty geographically defined areas in the Netherlands(Reference Herings, Bakker and Stricker26–Reference Hepburn, Howlett and Boeing28). Data used for the present study were the person's age and gender, the prescribed drug, the anatomical therapeutic chemical (ATC) classification, the defined daily dose (DDD)(29), the dispensing date and the amount dispensed.
Exposure definition
The FFQ of the Doetinchem Cohort Study contained an open question on the brand name of the spread used on bread. The amount of margarine used was calculated by multiplying the number of bread slices consumed daily by the amount of margarine per slice, estimated from photographs of four differently sized portions. On each examination day, users of phytosterols/-stanols were defined as those with an intake of phytosterol/-stanol-enriched margarine greater than zero. To evaluate the effects of different levels of margarine use, the average margarine intake was categorized into no, low (>0 to <10 g/d), medium (≥10 to <20 g/d) or high (≥20 g/d). This represents no, low (>0 to <0·75 g/d), medium (≥0·75 to <1·5 g/d) or high (≥1·5 g/d) intake of phytosterols/-stanols. From the pharmacy-dispensing records, all prescriptions for statins (ATC classification C10AA) dispensed between 1 January 1998 and 1 October 2008 were selected. The type and dose of statin used were converted into a single equipotency score according to Penning-van Beest et al.(Reference Penning-van Beest, Termorshuizen and Goettsch30). Subjects were considered to be users of statins at the examination day, re-examination day or both if they were, according to PHARMO-RLS, exposed to the drug on that specific day. Linking the Doetinchem Cohort data to PHARMO-RLS resulted in four categories of users on each examination day: (i) non-users; (ii) subjects using phytosterols/-stanols without statins; (iii) subjects using statins without phytosterols/-stanols; and (iv) subjects who combined phytosterols/-stanols and statins (combination users).
Outcome definition
TC and HDL-C were determined from non-fasting blood samples using standardized enzymatic methods(Reference Houterman, Verschuren and Oomen31). Non-HDL-C was calculated as the difference between TC and HDL-C. The effectiveness of the phytosterol/-stanol-enriched margarine and/or statins was assessed by the change in TC and non-HDL-C, and in the ratio of TC to HDL-C (TC:HDL-C), between the examination and the re-examination day.
Potential confounding variables
The following variables were considered as possible confounders: age, gender, BMI, waist:hip ratio (WHR), energy intake, (un)saturated and total fat intake, dietary cholesterol intake, alcohol intake, smoking behaviour, physical activity level, systolic and diastolic blood pressure, type 2 diabetes and educational level. Variables that altered the regression coefficient of the usage indicator variable by ≥10 % were entered in the model as confounding factors(Reference Greenland32).
Statistical analyses
General characteristics
Demographic and health characteristics of the four groups of users were compared using ANOVA or the Kruskal–Wallis test for continuous variables and the χ 2 test for nominal variables. Analyses were based on the re-examination data of the Doetinchem study (2003–2007), as the phytosterol/-stanol-enriched margarines were available on the Dutch market only from 1999 onwards.
Effectiveness of phytosterols/-stanols and statins
A general linear regression model was used to assess differences in TC change over time between subjects not using cholesterol-lowering products at any moment and subjects who started to use phytosterols/-stanols without statins, statins without phytosterols/-stanols or both compounds at some point in time between the two examination days (analysis I, see Fig. 1). Multivariate ANOVA was carried out to adjust for confounders at examination (1998–2002). All models were adjusted for cholesterol levels at examination as it has been shown that patients with high baseline cholesterol levels experience larger reductions in cholesterol levels after phytosterol/-stanol or statin intake(Reference Ketomaki, Gylling and Miettinen33). In order to describe the cholesterol-lowering effects of the use of phytosterols/-stanols more thoroughly, to include persons already using phytosterol/-stanol-enriched margarines in the years 1999–2002 and to be able to adjust for time-varying confounders, repeated-measures analysis of covariance (ANCOVA) was used (analysis II, see Fig. 1). The following fixed effects were included in the model: use of phytosterol/-stanol-enriched margarine, use of statin and time. Furthermore, an interaction term for enriched margarine and statins was entered in the model to test whether there was a difference between the effect of the enriched margarine given with statins and the effect of the enriched margarine given without statins. Use of enriched margarine was entered in the model as a dichotomous variable (yes/no), as a continuous variable (enriched margarine use in g/d) and as a categorical variable (no/low/medium/high intake). Models were checked for collinearity and residuals were checked for homoscedasticity, outliers and normal distribution. Non-HDL-C and TC:HDL-C were analysed in the same way.

Fig. 1 Flowchart of subject numbers in the linked database used for analysis I and II
P values were considered statistically significant at the 0·05 level. The Statistical Analysis Systems statistical software package version 9·1·3 (SAS Institute, Cary, NC, USA) was used for all analyses.
Results
General characteristics
From the linked database, complete records were available for 3829 subjects (Fig. 1). These subjects were examined in the Doetinchem Cohort Study during the years 1998–2002 and re-examined during the years 2003–2007. At re-examination, 195 (5·1 %) of these subjects used phytosterol/-stanol-enriched margarine only, whereas forty-three subjects (1·1 %) combined the use of enriched margarine with statins. A total of 303 subjects (7·9 %) used statins only.
Phytosterol/-stanol-enriched margarine, with or without statins, was more frequently used among the higher educated and phytosterol/-stanol users consumed more alcohol daily. The vast majority of subjects who used enriched margarine used phytosterol-enriched margarine (98 %). Median intake of margarine was 13 g/d, ranging from 0·12 g/d to 60 g/d (0·01 to 4·5 g phytosterols/-stanols per d). There was no significant difference in intake amount between subjects who did or did not combine their phytosterol/-stanol intake with statins. Only 9 % of the subjects used the recommended margarine intake of 27 g/d (2 g phytosterols/-stanols per d).
Statin users, whether or not in combination with phytosterols/-stanols, were more likely to be male, had higher WHR and perceived their health more often as moderate or poor compared with statin non-users. Users of cholesterol-lowering products, either phytosterol/-stanol-enriched margarine and/or statins, were older and consumed less dietary (saturated) fat compared with non-users (Table 1).
Table 1 Demographic and health characteristics of non-users, phytosterol/-stanol users, statin users and combination users in the linked database (n 3829)
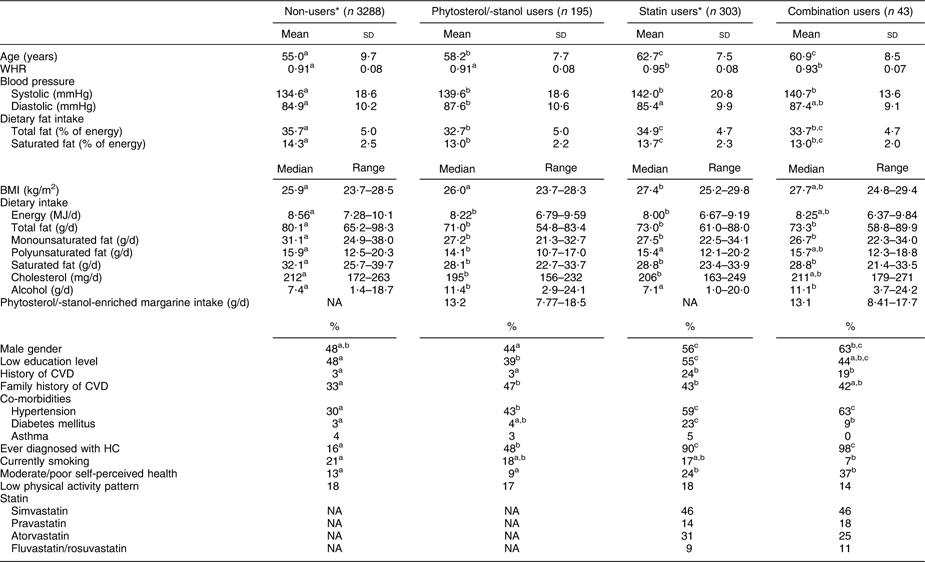
WHR, waist:hip ratio; HC, hypercholesterolemia; NA, not applicable.
a,b,cValues within a row with unlike superscripts were significantly different (P < 0·05).
*Numbers vary due to missing values.
Effectiveness of phytosterols/-stanols in statin users and statin non-users
Table 2 presents the results of the univariate and multivariate linear regression analysis (analysis I). Calculations are based on a total of 169 phytosterol/-stanol only users, 203 statin only users, twenty-four combination users and 3255 non-users. These persons did not use phytosterol/-stanol-enriched margarine or statins at examination (1998–2002) and started to use one or both of these products at some point in time during the 5-year interval until re-examination. From Table 2a it appears that at examination, thus before the start of phytosterols/-stanols and/or statins, mean serum TC levels of future phytosterol/-stanol-enriched margarine only users were significantly higher than those of non-users (6·15 mmol/l v. 5·62 mmol/l, P < 0·0001), but significantly lower than those of future statin only users (6·15 mmol/l v. 6·66 mmol/l, P < 0·0001) and combination users (6·15 mmol/l v. 6·69 mmol/l, P = 0·015). TC, non-HDL-C and TC:HDL-C decreased significantly during the 5-year follow-up period in all users, compared with the reference group (non-users; Table 2b). The largest difference in TC change compared with the non-users was found in combination users (−1·64 (95 % CI −1·91, −1·37) mmol/l), followed by statin only users (−1·40 (95 % CI −1·51, −1·30) mmol/l). Statistical significance was not reached for change in TC between these groups (P = 0·11), but there was a significant difference in change in non-HDL-C and TC:HDL-C between combination users and statin only users.
Table 2 (a) Serum cholesterol levels at examination (1998–2002) and (b) change in cholesterol levels between examination (1998–2002) and re-examination (2003–2007) day in subjects who started to use phytosterols/-stanols without statins (n 169), statins without phytosterols/-stanols (n 203) or a combination of both compounds (n 24) between examination and re-examination, as compared with subjects who did not start using phytosterols/-stanols or statins (non-users, n 3255) (analysis I). Data from the linked database
(a) Serum cholesterol levels at examination (1998–2002)
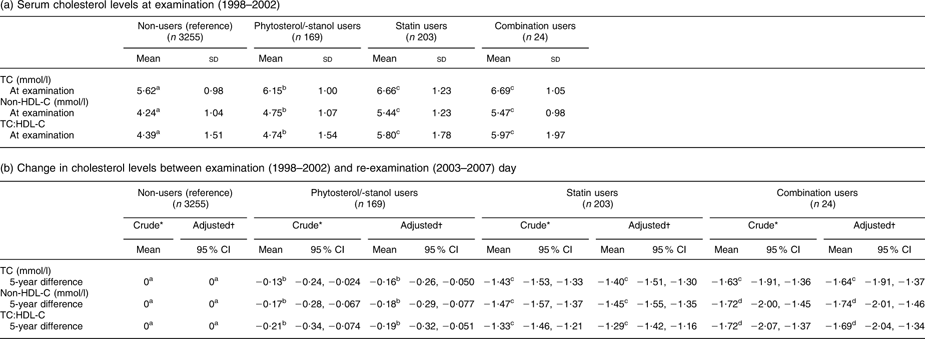
TC, total cholesterol; non-HDL-C, non-HDL cholesterol.
To convert cholesterol mmol/l to mg/dl, multiply by 38·7.
a,b,c,dMean values within a row with unlike superscripts were significantly different (P < 0·05).
*Adjusted for cholesterol level at examination.
†Adjusted for age, BMI, waist:hip ratio, saturated fat intake, alcohol intake, diastolic blood pressure, type 2 diabetes and cholesterol level at examination.
Results of the repeated-measures ANCOVA are shown in Table 3 (analysis II). After adjustment for age, BMI, WHR, saturated fat intake, alcohol intake, diastolic blood pressure, type 2 diabetes and statin use, the intake of phytosterols/-stanols was significantly associated with a decrease in TC of −0·11 (95 % CI −0·20, −0·025) mmol/l. Similarly, non-HDL-C and TC:HDL-C decreased significantly over time when phytosterols/-stanols were used. There was no evidence of an interactive effect between enriched margarine use and statin use, as the interaction term was not significant.
Table 3 Effectiveness of phytosterols/-stanols on change in TC, non-HDL-C and TC:HDL-C between examination (1998–2002) and re-examination (2003–2007), according to repeated-measures analysis of covariance (analysis II). Data from the linked database
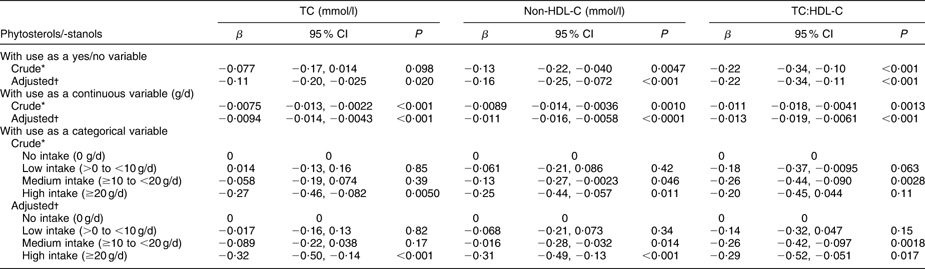
TC, total cholesterol; non-HDL-C, non-HDL cholesterol.
To convert cholesterol mmol/l to mg/dl, multiply by 38·7.
*Adjusted for equipotency score of statin.
†Adjusted for age, BMI, waist:hip ratio, saturated fat intake, alcohol intake, diastolic blood pressure, type 2 diabetes and equipotency score of statin.
Each gram increase in enriched margarine use resulted in a decrease in TC of −0·0094 (95 % CI −0·014, −0·0043) mmol/l. Also non-HDL-C and TC:HDL-C were significantly reduced by phytosterols/-stanols. The effectiveness of phytosterols/-stanols to lower TC increased progressively across the four categories of intake amounts (0; −0·017 (95 % CI −0·16, 0·13) mmol/l; −0·089 (95 % CI −0·22, 0·038) mmol/l; −0·32 (95 % CI −0·50, −0·14) mmol/l). Similar patterns were found for non-HDL-C and TC:HDL-C, although these outcome measures were significantly reduced following an intake of ≥10 g enriched margarine per d, whereas TC was significantly reduced only after high intake (≥20 g/d).
Discussion
Our results indicate that the use of margarine enriched with phytosterols/-stanols is effective in lowering TC, non-HDL-C and TC:HDL-C in both users and non-users of statins under free-living conditions. In the present study, serum TC decreased by respectively 0·16 mmol/l, 1·40 mmol/l and 1·64 mmol/l in subjects who started to use phytosterols/-stanols only, statins only or a combination of both compounds at some point in time between the examination and re-examination day, compared with subjects who did not start using phytosterols/-stanols or statins. Statistical significance was not reached for change in TC between combination users and statin only users (P = 0·11), but there was a significant difference in change in non-HDL-C and TC:HDL-C between these groups. Repeated-measures ANCOVA showed slightly lower levels of effectiveness, most likely explained by the fact that the greatest reductions in cholesterol levels are achieved in subjects who started the use of the enriched margarine. The cholesterol-lowering effect of the margarine when added to statin therapy was similar to the effect observed when the margarine was used alone. This additive effect of the enriched margarine to statin therapy has also been found in prior studies(Reference de Jong, Zuur and Wolfs34, Reference Simons35). Intake amounts above 20 g margarine/d (1·5 g phytosterols/-stanols per d) were necessary to reduce TC significantly. In our study, only 20 % of the subjects used this intake level.
In the model with continuous variables, each gram intake of margarine was associated with reductions in TC of 0·0094 mmol/l. People who consume the recommended intake of enriched margarine of 27 g/d (2 g phytosterols/-stanols per d) may reduce their TC level by 0·25 mmol/l (0·0094 mmol/l × 27 g), which is about 4 %. Although no trials have investigated the direct relationship between the intake of phytosterols/-stanols and CHD risk reduction, data from RCT and prospective studies indicate that a 4 % decrease in serum TC levels would reduce the incidence of CHD by approximately 10–15 %(36, Reference Law, Wald and Thompson37). A recently conducted meta-analysis of RCT on the LDL-C-lowering effects of phytosterols/-stanols found that LDL-C was reduced by 0·34 mmol/l (or 8·8 %) for a daily intake of 2·15 g phytosterols/-stanols(Reference Demonty, Ras and van der Knaap16). In the present study, a daily intake of 2 g phytosterols/-stanols reduced LDL-C levels by approximately 0·25 mmol/l or 5 %, given that the cholesterol-lowering effect of phytosterols/-stanols affects only LDL-C and about 80 % of the circulating cholesterol in the human body is carried bound to LDL(Reference Crowley38). This level of effect is considerably lower than the effects expected from RCT. As could be expected, effects from statins were substantially larger compared with the effects achieved by the use of enriched margarines.
In this Dutch cohort, 98 % of the enriched margarine users consumed phytosterol-enriched margarine. Yet, it is reasonable to assume that our results are also applicable to countries or situations where phytostanols are more commonly used. Phytosterols and -stanols have been found to reduce cholesterol levels equally in both short(Reference Hallikainen, Sarkkinen and Gylling39–Reference O'Neill, Brynes and Mandeno42) and longer(Reference de Jong, Plat and Ltjohann43) term RCT(Reference Demonty, Ras and van der Knaap16), albeit it has been suggested that the cholesterol-lowering effect of phytosterols attenuates over time due to down-regulation of bile acid synthesis(Reference O'Neill, Sanders and Thompson44).
Two other related studies explored the effectiveness of phytosterols/-stanols and statins in a real-life setting(Reference de Jong, Zuur and Wolfs34, Reference Wolfs, de Jong and Ocke45). The first study did not find any significant differences in effects between cholesterol-lowering drugs only and combined intake(Reference Wolfs, de Jong and Ocke45). On the other hand, phytosterols/-stanols appeared to reduce cholesterol levels additively to cholesterol-lowering drugs in the second study(Reference de Jong, Zuur and Wolfs34). A major limitation of these studies was the small number of combination users; only twelve and fifteen subjects combined enriched margarine and cholesterol-lowering drugs in the first and second study, respectively. Moreover, those studies did not distinguish between statins and other cholesterol-lowering drugs and questionnaires were used for the determination of drug usage. Administrative databases, such as PHARMO-RLS, have the advantage that patient-related recall bias and non-response bias are reduced, precise information about prescribed drugs can be obtained and the drug history is available over a long period. Pharmacy data have the advantage over medical records of being able to obtain information regarding what medication was acquired instead of what medication was prescribed. However, uncertainty still exists concerning whether or not the drug is actually taken. Another limitation of the present study is that no information was available about the use of other phytosterol/-stanol-containing products. This might have led to an overestimation of the effect of phytosterol/-stanol-enriched margarines, as phytosterol/-stanol-enriched margarine users might be inclined to use other phytosterol/-stanol-enriched products as well. In addition, no information on food intake was gathered in the 5-year interval between examination and re-examination and it should be acknowledged that this is an observational study which might be subject to residual confounding due to potential unmeasured differences in cardiovascular risk profile and patient characteristics between users and non-users of phytosterol/-stanol-enriched margarine and/or statins. The restriction of the present study to a particular area of the Netherlands might constrain the generalisability of the results. Doetinchem is a rural area in the eastern part of the Netherlands and smokers and the lower educated appear to be under-represented in the cohort. However, although it is conceivable that this affects the number of subjects using enriched margarine or the baseline lipid values, it is unlikely that it has an influence on the estimated associations.
For the purpose of the study, a database was used which included pharmacy-dispensing data and questionnaire data on health and food intake. There are no standard databases available that integrate food and drug monitoring, and thus methods that link large health survey data and pharmacy data are necessary to investigate effects of a combination of (functional) foods and drugs. By using such databases items like type of consumers, overall effectiveness of therapies, adherence to food and drug therapies, potential interactions on a behavioural or physiological level and long-term safety can be studied. In the near future this will become more and more important because the market for functional foods and dietary supplements with a health claim is expanding rapidly worldwide and consequently an increasing number of persons will use these products and combine them with their prescribed drugs.
Conclusions
In the present study we found that phytosterol/-stanol-enriched margarine is effective in lowering TC, non-HDL-C and TC:HDL-C under customary conditions in both statin users and statin non-users. Recommended intake levels were achieved by only 9 % of the subjects and resulted in a 4 % decline in TC levels. Phytosterol/-stanol-enriched margarine can be recommended to statin non-users with normal to moderately increased serum TC and non-HDL-C concentrations who wish to maintain their cholesterol levels at, or reduce their cholesterol levels to, healthy levels. Statin users who wish to reduce their TC and non-HDL-C levels through diet can use the phytosterol/-stanol-enriched margarines as an adjunct to their ongoing statin therapy. This might be especially beneficial for those subjects who do not achieve recommended TC and non-HDL-C target levels with statin monotherapy. Dietetics professionals should advise consumers on the appropriate intake level of the enriched margarines and should teach consumers how to use phytosterol/-stanol-enriched margarine as part of a balanced diet.
Acknowledgements
The study was funded by a grant from the National Institute for Public Health and the Environment (RIVM). The division of Pharmacoepidemiology and Clinical Pharmacology employing authors S.R.B.M.E. and O.H.K. has received unrestricted funding for pharmacoepidemiological research from GlaxoSmithKline, Novo Nordisk, the private–public funded Top Institute Pharma (www.tipharma.nl, includes co-funding from universities, government and industry), the Dutch Medicines Evaluation Board and the Dutch Ministry of Health. The authors N.d.J., C.J.M.R., J.G. and W.M.M.V. have no conflicts of interest to disclose. The Dutch Doetinchem Cohort Study was financially supported by the Ministry of Public Health, Welfare and Sports of the Netherlands and the National Institute of Public Health and the Environment (RIVM), Bilthoven. S.R.B.M.E., W.M.M.V. and O.H.K. designed the research. S.R.B.M.E. and O.H.K. analysed the data. All authors wrote the paper. S.R.B.M.E. and O.H.K. had primary responsibility for the final content. All authors read and approved the final manuscript. The authors thank the epidemiologists and field workers of the Municipal Health Service in Doetinchem for their contribution to the data collection for this study. W.M.M.V. was Project Leader; data management was performed by A. Blokstra MSc, P.E. Steinberger MSc and A.W.D. van Kessel MSc; logistic support was provided by J. Steenbrink-van Woerden MSc and P. Vissink; and secretarial assistance was provided by E. van der Wolf.






