Orofacial clefts (OFC) are among the most common birth defects worldwide, constituting the main disorders affecting craniofacial structures( Reference Mossey 1 ). Their prevalence shows variation according to factors such as ethnic origin and socio-economic status( 2 , Reference Watkins, Meyer and Strauss 3 ). OFC have been classified as cleft palate only (CPO), cleft lip only (CL) and cleft lip with cleft palate (CLP). These latter two categories are grouped as cleft lip with or without cleft palate (CL/P)( Reference Schutte and Murray 4 ). About 70 % of OFC occur as a non-syndromic condition (NSOFC) without any other apparent structural or cognitive abnormality, while the remaining 30 % are found as part of more than 300 recognizable genetic syndromes( Reference Watkins, Meyer and Strauss 3 , Reference Schutte and Murray 4 ). OFC constitute a worldwide public health problem due to their prevalence, complex rehabilitation plus medical costs, and emotional burden to patients and their families. In this context, OFC patients present a wide variety of medical complications in early processes such as feeding, speaking and hearing and in their social integration( Reference Dixon, Marazita and Beaty 5 ). In addition, these patients have a higher risk of certain cancers and psychiatric disorders in adult life( Reference Vieira 6 ).
The aetiology of OFC can be explained by the interaction between functionally altered genes plus environmental factors( Reference Dixon, Marazita and Beaty 5 ). Probably the best example of a gene–environmental interaction in clefts’ aetiology is folate/folic acid (FA) metabolism( Reference Bhaskar, Murthy and Venkatesh Babu 7 ). Folates are involved in the transfer of methyl groups (one-carbon units) to DNA, being an epigenetic mechanism of gene expression modulation( Reference Lucock 8 , Reference Jones and Takai 9 ). Maternal folate/methyl-donor status appears to play a central role during early embryonic development where its deficit affects embryo and fetal cells with high proliferation rates such as neural crest cells( Reference Bhaskar, Murthy and Venkatesh Babu 7 ). These cells notably contribute to maxillofacial bone and cartilage development by means of an epigenetic-regulated differentiation( Reference Jones and Takai 9 ). Subsequently, epigenetic mechanisms modulate the secondary palate development as has been demonstrated in animal models( Reference Bogdanović and Veenstra 10 ). Compared with mothers of non-affected children, mothers of cleft cases had a diet poor in folates( Reference Figueiredo, Figueiredo and Feguri 11 ). In this context, multi-ethnic systematic reviews have demonstrated that maternal periconceptional FA supplementation is an effective intervention preventing OFC( Reference Badovinac, Werler and Williams 12 – Reference Molina-Solana, Yáñez-Vico and Iglesias-Linares 14 ) while other authors showed a neutral pooled effect( Reference De-Regil, Peña-Rosas and Fernández-Gaxiola 15 ). Thus, the FA–OFC relationship is still an issue that needs further studies.
In January 2000, the Chilean Ministry of Health established a mandatory fortification of wheat flour with FA( Reference Cortes, Mellado and Pardo 16 ). This national public health policy led to a significant reduction (about 50 %) in the prevalence of neural tube defects( Reference López-Camelo, Orioli and da Graça Dutra 17 , Reference López-Camelo, Castilla and Orioli 18 ). Despite the above-mentioned role of FA supplementation in preventing OFC, in several countries the effect of FA fortification on its prevalence is controversial. Some studies in the Chilean population did not show benefits on OFC reduction( Reference López-Camelo, Castilla and Orioli 18 , Reference Castilla, Orioli and Lopez-Camelo 19 ); however, other authors registered no effects for CL/P but a significant increase in CPO prevalence in the country( Reference Nazer, Cifuentes and Aguila 20 , Reference Nazer and Cifuentes 21 ). On the other hand, in Iran a significant decrease of NSOFC was observed as a response to the mandatory FA fortification( Reference Golalipour, Vakili and Kaviani 22 ). Therefore we aimed to perform a meta-analysis based on published studies comparing prevalence rates for all OFC types in FA pre-fortification v. post-fortification periods in order to contribute to solving this controversy.
Methods
Literature search and quality assessment of single studies
In order to minimize the risk of bias, we performed a search based on the following considerations. (i) A search was conducted in diverse conventional scientific literature databases: Cochrane Library, EMBASE, PubMed, ScienceDirect, Scielo, Springerlink and Web of Science; and in grey literature databases: GreyNet, GreyLit, LILACS, Open Grey and POPLINE (Fig. 1). The literature search was conducted through 15 August 2016 with no date restrictions for early studies, considered the terms ‘cleft lip palate’ OR ‘cleft palate’ OR ‘orofacial clefts’ AND ‘folic acid’, and was restricted to English and Spanish languages. (ii) The search was performed independently by two authors (N.M. and J.S.) who extracted the following data from each report: authors, year of publication, country of origin of the samples, number of OFC cases and total births for pre-fortification period, and number of OFC cases and total births for post-fortification period. (iii) For samples from the same country/region/state, we confirmed that different time lapses for pre- and/or post-fortification periods were considered. (iv) We performed a quality assessment for each study identified using the Newcastle–Ottawa Scale (NOS), considering the selection criteria of pre- and post-fortification samples, comparability of these groups, and the ascertainment of either the exposure or outcome. NOS assigns a maximum score of 9 points where studies showing <5 points have high risk of bias and limitations, with these being excluded from a meta-analysis( Reference Wells, Shea and O’Connell 23 ).

Fig. 1 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart showing selection of studies for the present meta-analysis on the effects of folic acid (FA) fortification on orofacial clefts (OFC) prevalence
Statistical methods
Meta-analysis was performed comparing OFC prevalence between pre- and post-fortification periods considering all births in a region, state or hospitals within a country. The effect was estimated by means of the relative risk (RR) with 95 % CI for each study and for the pooled effect. The presence of between-study heterogeneity was evaluated by the Cochran Q statistic, which is the base for the I 2 test expressing the percentage of between-study variability explained by heterogeneity( Reference Huedo-Medina, Sánchez-Meca and Marín-Martínez 24 ). The pooled effect was estimated using fixed-effects or random-effects methods based on the absence (I 2<50) or presence of heterogeneity (I 2>50), respectively( Reference Borenstein, Hedges and Higgins 25 ). In the presence of heterogeneity and in order to identify its sources, we additionally applied a univariate meta-regression( Reference Borenstein, Hedges and Higgins 25 ) between each study effect and three covariates: total sample size, the time lapse (months) between the pre- and post-fortification, and the FA mean daily dose reached by fortification in each country based on the Food Fortification Initiative( 26 ). Publication bias (i.e. studies which have been published based on their sample size and their positive or negative effect) was evaluated using Begg’s funnel plot( Reference Borenstein, Hedges and Higgins 25 ). If a meta-analysis includes studies of mainly small sample size and/or a low number of studies (≤10), the funnel-based method loses power( Reference Coburn and Vevea 27 ). Therefore, in this case, publication bias was alternatively assessed via cumulative meta-analysis by precision( Reference Coburn and Vevea 27 ). A sensitivity analysis was performed to assess the robustness of meta-analysis based on the detection of significant changes related to the overall effect when one study is dropped at a time and the pooled effect is recalculated (leave-one-out method)( Reference Borenstein, Hedges and Higgins 25 ). All tests were performed using the statistical OpenMeta (Analyst) package( Reference Wallace, Dahabreh and Trikalinos 28 ).
Results
Figure 1 summarizes the steps and results of our search in order to find a group of publications complying with the criteria to achieve the aim of our study and with proper quality. The initial search showed a total of 961 reports. After discarding duplicated studies, we evaluated 653 reported abstracts. Then, 356 articles met the criteria to be analysed at full text level. Finally, fifteen studies were considered in the quality analysis (NOS) and all of them were included for pooled effects of FA fortification on OFC risk. Data extracted from the selected articles are detailed in Table 1( Reference López-Camelo, Castilla and Orioli 18 – Reference Golalipour, Vakili and Kaviani 22 , Reference Ray, Meier and Vermeulen 29 – Reference Yang, Carmichael and Shaw 38 ). If a study included samples from two or more countries, these samples were evaluated separately.
The first analysis included all OFC considering the fifteen studies selected where syndromic and non-syndromic cases were grouped. These reports comprised twenty-four samples from different countries or regions in a same country (Table 1). We detected between-study heterogeneity (I 2=78·5 %; Fig. 2). Thus, a random-effects model meta-analysis was applied, which showed a non-significant influence of FA fortification on OFC prevalence (RR=0·97; 95 % CI 0·92, 1·02; Fig. 2). Univariate meta-regression recognized the additional FA daily dose reached by fortification as a source of heterogeneity (regression coefficient=−0·576; 95 % CI −1·022, −0·130; see online supplementary material, Table S1). The funnel plot exhibited asymmetry (see online supplementary material, Fig. S1(a)), demonstrating the presence of publication bias. Sensitivity analysis supported the robustness of the meta-analysis results (data not shown). Then, we identified the studies where CL/P and CPO data were analysed separately (ten articles including sixteen samples). For CL/P we found evidence of heterogeneity (I 2=79·5 %; Fig. 3) and the pooled effect based on a random-effects model showed no significant effect of fortification (RR=0·99; 95 % CI 0·92, 1·06; Fig. 3). Univariate meta-regression did not detect heterogeneity sources (Table S1). Cumulative meta-analysis by precision demonstrated the absence of publication bias (Fig. S1(b)). However, the sensitivity analysis showed that our results lacked robustness, where the exclusion of samples from Texas( Reference Hashmi, Waller and Langlois 32 ) and Brazil( Reference López-Camelo, Castilla and Orioli 18 ) respectively significantly decreased (RR=0·73; 95 % CI 0·67, 0·80) and increased CL/P risk (RR=1·32; 95 % CI 1·09, 1·60; data not shown). Fortification also showed a non-significant effect on CPO prevalence based on a random-effects model (I 2=90·2 %; RR=1·02; 95 % CI 0·89, 1·18; Fig. 4). This heterogeneity could not be explained by any of the analysed covariates (Table S1). Sensitivity analysis demonstrated the robustness of our results (data not shown) but cumulative meta-analysis by precision showed evidence of publication bias (Fig. S1(c)).
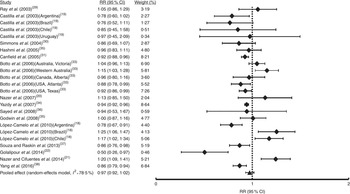
Fig. 2 Forest plot showing the effect of folic acid fortification on the prevalence of total orofacial clefts (total OFC). The study-specific relative risk (RR) and 95 % CI are represented by the black square and horizontal line, respectively; the black triangle represents the pooled RR and the horizontal line represents the pooled 95 % CI; the dotted line at RR=1 represents the null effect

Fig. 3 Forest plot showing the effect of folic acid fortification on the prevalence of cleft lip with or without cleft palate (CL/P). The study-specific relative risk (RR) and 95 % CI are represented by the black square and horizontal line, respectively; the black triangle represents the pooled RR and the horizontal line represents the pooled 95 % CI; the dotted line at RR=1 represents the null effect

Fig. 4 Forest plot showing the effect of folic acid fortification on the prevalence of cleft palate only (CPO). The study-specific relative risk (RR) and 95 % CI are represented by the black square and horizontal line, respectively; the black triangle represents the pooled RR and the horizontal line represents the pooled 95 % CI; the dotted line at RR=1 represents the null effect
Table 1 Description of the studies and samples in the present meta-analysis on the effects of folic acid (FA) fortification on orofacial clefts prevalence

Pre-F, FA pre-fortification period; Post-F, FA post-fortification period; NS(S), studies where non-syndromic and syndromic clefts are recognizable; CL/P(CPO), studies where cleft lip with or without cleft palate (CL/P) and cleft lip palate only (CPO) are recognizable; NOS score, score from Newcastle–Ottawa Scale( Reference Wells, Shea and O’Connell 23 ).
Only in five studies (comprising seven samples) was it possible to identify non-syndromic cases from syndromic cases of OFC (Table 1). Random-effects meta-analysis showed that FA fortification had no effect on NSOFC prevalence (I 2=54·6 %; RR=0·92; 95 % CI 0·83, 1·03; Fig. 5). This heterogeneity may be explained by the total sample size of each study, which was negatively associated with the effect (regression coefficient=−0·004; 95 % CI −0·003, −0·005; Table S1). Sensitivity analysis showed that upon exclusion of the report from Ontario( Reference Ray, Meier and Vermeulen 29 ) the effect of FA fortification became significant (RR=0·90; 95 % CI 0·82, 0·98; data not shown). There was no substantial evidence of publication bias from cumulative meta-analysis by precision (Fig. S1(d)). Only three studies (five samples) considered NSCL/P and NSCPO classification. Fixed-effects model meta-analysis for NSCL/P demonstrated a significant reduction of its risk after FA fortification (I 2=22·4 %; RR=0·88; 95 % CI 0·81, 0·96; Fig. 6). These results are supported by both the absence of publication bias according to the cumulative meta-analysis (Fig. S1(e)) and the robustness of our findings based on the sensitivity analysis (data not shown). On the contrary, NSCPO meta-analysis exhibited heterogeneity and no significant effect of FA fortification on its prevalence (I 2=71·1 %; RR=1·00; 95 % CI 0·75, 1·34; Fig. 7). We did not find sources for this heterogeneity among the covariates considered for meta-regression (Table S1). Cumulative meta-analysis by precision showed no conclusive evidence of publication bias for NSCPO (Fig. S1(e)), while sensitivity analysis demonstrated the robustness of this last meta-analysis (data not shown).

Fig. 5 Forest plot showing the effect of folic acid fortification on the prevalence of non-syndromic orofacial clefts (NSOFC). The study-specific relative risk (RR) and 95 % CI are represented by the black square and horizontal line, respectively; the black triangle represents the pooled RR and the horizontal line represents the pooled 95 % CI; the dotted line at RR=1 represents the null effect

Fig. 6 Forest plot showing the effect of folic acid fortification on the prevalence of non-syndromic cleft lip with or without cleft palate (NSCL/P). The study-specific relative risk (RR) and 95 % CI are represented by the black square and horizontal line, respectively; the black triangle represents the pooled RR and the horizontal line represents the pooled 95 % CI; the dotted line at RR=1 represents the null effect

Fig. 7 Forest plot showing the effect of folic acid fortification on the prevalence of non-syndromic cleft palate only (NSCPO). The study-specific relative risk (RR) and 95 % CI are represented by the black square and horizontal line, respectively; the black triangle represents the pooled RR and the horizontal line represents the pooled 95 % CI; the dotted line at RR=1 represents the null effect
Discussion
Our study presents certain characteristics which may be considered as strengths: a wide bibliographical search in several medical/scientific conventional and grey databases, where all of the included reports showed a high quality level (Table 1). Thus, these features allow us to consider that all studies included in our meta-analysis have a low risk of bias. On the other hand, the great majority of our analyses exhibited between-study heterogeneity (with the exception of NSCL/P meta-analysis), results reflecting a limitation of the present report. However, we decided to search for sources of this heterogeneity instead of not considering these pooled effects. Univariate meta-regression discovered the possible heterogeneity sources in some cases (Table S1). For all OFC, FA dose was negatively associated with cleft risk, which is related to evidence showing a decrease in cleft occurrence by periconceptional FA use in a dose-dependent manner( Reference Tolarova 39 , Reference Czeizel, Tímár and Sárközi 40 ). Additionally, for NSOFC we found that between-study heterogeneity may be explained by the sample size of each study. Sample size variation (directly associated to the precision of each study) is always an important factor affecting heterogeneity in meta-analysis( Reference Higgins 41 ). The between-study variability detected could also be explained by variables not included in the meta-regression, such as the ethnic origin of the populations. Samples considered herein came from South and North America, Africa, Asia and Oceania. In this context, the response to FA consumption may be modulated by variants in folate/one-carbon metabolism genes( Reference Bhaskar, Murthy and Venkatesh Babu 7 ) such as the functional polymorphism 677C→T within the MTHFR gene coding a central enzyme (methylenetetrahydrofolate reductase) in this metabolism( Reference Matthews, Sheppard and Goulding 42 ). Its allelic frequency differs according to the ethnicity of each population (based on dbSNP information)( 43 ). We decided to not perform meta-analysis segregating samples for continent in order to avoid a decrease in statistical power associated with forming subgroups. For total OFC and CPO, we found evidence of publication bias; this is another limitation. In this context, the report of Castilla et al. ( Reference Castilla, Orioli and Lopez-Camelo 19 ), using samples from the Latin American Collaborative Study of Congenital Malformations (ECLAMC), excluded samples from certain countries where a low number of annual births (<10 000) was registered in the participating hospitals, contributing to this bias.
We applied the NOS in order to detect low-quality reports which have high risk of bias( Reference Wells, Shea and O’Connell 23 ). However, this scale was designed to be applied on case–control or cohort studies. As was commented by Simmons et al. ( Reference Simmons, Mosley and Fulton-Bond 30 ), all reports included here are considered ecological studies which may introduce an ecological bias possibly not detected by the NOS. A bias associated with this class of design is related to the latency time since exposure and effect measurement( Reference Borja-Aburto 44 ). In the current report, some studies did not consider a time lapse between pre- and post-fortification periods while other authors only included births registered months or years after the implementation of the mandatory FA policy (Table 1). Although this covariate was not detected as a source of heterogeneity when a meta-regression was applied (Table S1), it may introduce variability because the additional dose of FA does not homogeneously reach all people following application of the policy( Reference Castilla, Orioli and Lopez-Camelo 19 ).
Regarding the effects of FA fortification policy, our meta-analysis found a beneficial effect only for NSCL/P prevalence (Fig. 6) and neutral influences on the other classifications of clefts. Although the NSCL/P analysis considered a reduced number of studies (three studies with five samples), we found support for the relative robustness of our results. Thus, we did not find evidence of between-study heterogeneity (Fig. 6) or publication bias (Fig. S1(e)), and the combined effect remained consistent when any sample was dropped in the sensitivity analysis (data not shown). The different NSCL/P results in comparison with the other cleft categories may be explained by several factors. First, total OFC include both syndromic and non-syndromic forms. The great majority of the syndromic forms have known causes associated with teratogens, single-locus mutations and chromosomal rearrangements( Reference Leslie and Marazita 45 ). Thus, the inclusion of syndromic clefts may lead our results to neutral effects of FA. However, FA fortification did not show a significant effect on NSOFC prevalence, possibly as a consequence of an admixture of NSCPO and NSCL/P, which have been considered different aetiological entities. Epidemiological data show a higher frequency of CL/P in males than females, which is inverted for CPO( Reference Watkins, Meyer and Strauss 3 ). In addition, CPO is more frequent in syndromes than CL/P (50 and 30 %, respectively)( Reference Watkins, Meyer and Strauss 3 ). These differences seem to reflect the genetic and embryological origins of these clefts, where CL/P is associated with primary palate development whereas CPO is related to secondary palate closure, both considered independent events( Reference Watkins, Meyer and Strauss 3 , Reference Carinci, Pezzetti and Scapoli 46 ).
To the best of our knowledge, there is only one previous meta-analysis( Reference Johnson and Little 47 ) analysing the influence of FA fortification on OFC prevalence (grouping syndromic and non-syndromic forms). That meta-analysis considered only three studies which have also been included in the current report( Reference Simmons, Mosley and Fulton-Bond 30 , Reference Canfield, Collins and Botto 31 , Reference Botto, Lisi, Bower and Canfield 33 ). The previous meta-analysis found a significant but marginal decrease in the risk of CL/P after FA fortification which was not observed for CPO, supporting the hypothesis of a different aetiology among them. Those authors concluded that FA appears to play a minor or any role in OFC expression. Although we included a higher number of studies, our results lead us to the same conclusion as Johnson and Little( Reference Johnson and Little 47 ).
In summary, we found a significant decrease in the risk of NSCL/P after FA fortification in the current multi-ethnic meta-analysis. Although the number of NSCL/P samples was lower than that of total OFC, the absence of both between-study heterogeneity and publication bias, plus the robustness of this result in the sensitivity analysis, leads us to conclude that our finding is evidence of the beneficial effect of FA fortification policies.
Acknowledgements
Financial support: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors. Conflict of interest: All of the authors declare no conflict of interest. Authorship: N.M. and J.S. designed the study, performed the bibliographical search and data extraction. J.S. performed the statistical analyses. N.M., R.P., L.C. and J.S. (i.e. all authors) wrote, revised and approved the final version of the manuscript. Ethics of human subject participation: Not applicable.
Supplementary Material
To view supplementary material for this article, please visit https://dx.doi.org/10.1017/S1368980017000878











