Spontaneous abortion (SA), defined as the involuntary termination of a pregnancy at less than 28 completed weeks of gestation(Reference Neugebauer, Kline and Shrout1), is one of the most common adverse pregnancy outcomes. It is estimated that approximately 31 % of all clinically recognised pregnancies end as SA(Reference Wilcox, Weinberg and O’Connor2), and the rate of pregnancy loss prior to implantation is about 40 %, and they are often ignored as conceptions(Reference Jauniaux and Burton3). The rate of SA has been reported to be increased in the USA from 1970 to 2000(Reference Lang and Nuevo-Chiquero4), and reproductive-aged women increased from 1976 to 1997 in China(Reference Liu and Gao5). SA can increase the risk of recurrent miscarriage(Reference Xu, Wu and Yang6) and multiple psychological problems, such as depression and suicidal behaviours(Reference Kulathilaka, Hanwella and de Silva7), bringing huge physical damage to women and economic burden to families. Although at least half of the lost pregnancies, especially those in the first trimester, were derived from chromosomal abnormalities(Reference Cunningham, Leveno and Bloom8), the identification of other risk factors of SA is still crucial for risk-specific prevention.
Previous studies have identified various factors being related to SA, including infection, chemical toxicity exposure, immune dysfunction, endocrine disturbances, maternal disease and unhealthy lifestyle(Reference Xu, Wu and Yang6,Reference Larsen, Christiansen and Kolte9,Reference Wang, Yang and Liu10) . However, half of the reported lost pregnancies still cannot be clearly explained(Reference Xu, Wu and Yang6,Reference Vitzthum, Spielvogel and Thornburg11) . To date, maternal anaemia or high Hb concentration during pregnancy had been reported to have adverse effects on pregnancy outcomes(Reference Ren, Wang and Ye12–Reference Nair, Choudhury and Choudhury16). Studies showed that fetal death(Reference Nair, Choudhury and Choudhury16), stillbirth(Reference Nair, Churchill and Robinson15), intrauterine growth restriction(Reference Ronnenberg, Wood and Wang17), medical abnormalities of the fetus(Reference Williams and Wheby18) and even previous miscarriage history can be associated with anaemia(Reference Tandu-Umba and Mbangama19), which implied that maternal anaemia can be related to SA; however, the existing evidence of the relationship between maternal Hb concentrations and SA risk is conflicting(Reference Abeysena, Jayawardana and de Seneviratne20,Reference Buzyan21) .
Over 80 % of the lost pregnancies occur in the first trimester, while most women are unaware of their Hb levels until the first antenatal medical examination usually conducted during 8–12 gestational weeks. Thus, identifying the association of Hb concentration prior to pregnancy with the risk of SA is much more important to take preconception healthcare and reduce adverse pregnancy outcomes initiated before conception. Previous studies suggested maternal preconception anaemia is associated with increased risk of low birth weight, fetal growth restriction and small-for-gestational-age(Reference Ronnenberg, Wood and Wang17,Reference Yi, Han and Ohrr22) , and both preconception anaemia and high Hb concentration can significantly increase the risk of very preterm birth(Reference Zhang, Xu and Yang23). However, the relationship between preconception anaemia or high Hb concentration and SA risk was not well investigated. Therefore, we conducted a large population-based retrospective cohort study among over 3·9 million women in rural China to comprehensively examine the association between maternal Hb concentrations prior to pregnancy and the risk of SA.
Methods
Study design and participants
A large population-based retrospective cohort study among reproductive-aged (20–49 years) women was conducted based on National Free Pre-pregnancy Check-up Project (NFPCP), which was supported by the National Health Commission and Ministry of Finance of the People’s Republic of China, with the purpose to provide free preconception health examinations and follow-up of pregnancy outcomes for reproductive-aged couples who planned to get pregnant within the next 6 months. Published articles detailing the project-related design, organisation and implementation can be found elsewhere(Reference Wang, Yang and Liu10,Reference Zhang, Xu and Yang23,Reference Zhang, Wang and Sheng24) . The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving humans were approved by the Institutional Research Review Board at the National Research Institute for Health and Family Planning. Written informed consent was obtained from all NFPCP participants.
A total of 4 842 176 women aged 20–49 years participated in the NFPCP from 1 January 2013 to 31 December 2016 and successfully got pregnant and completed pregnancy outcomes follow-up until 31 December 2017, in rural China. We then excluded 870 748 women who had sexually transmitted disease or took medication at baseline examination, failed to get pregnant within 6 months and ended up with ectopic pregnancy or multiple births. As a result, 3 971 428 women were finally included in the primary analysis. Detailed information on the study population recruitment, and derivation of the population used in the final analysis, is shown in Fig. 1.

Fig. 1 Flowchart of the participants. *Sexually transmitted disease including infection of gonorrhoea, syphilis and chlamydia
Data collection
Briefly, NFPCP includes three stages: preconception health examination, early pregnancy follow-up and pregnancy outcome follow-up. Couples intending to conceive in the next 6 months were encouraged to take part in NFPCP by the local resident committee. Baseline information, including demographic characteristics, lifestyle, history of chronic diseases, reproductive history, medication history, toxicants exposure and dietary habit, was collected by trained health staff in the local maternal and child healthcare service centres in each county using a standard and structured questionnaire through face to face interview. Then physical examination was conducted by the trained health staff. Blood samples were drawn and stored at 4–8°C, then sent to the local laboratories. The National Center of Clinical Laboratories for Quality Inspection and Detection was responsible for the external quality assessment biannually, and for quality control(Reference Wang, Zhang and Zhang25). All data were uploaded and transferred remotely and stored in the NFPCP medical service information system.
After preconception health examination, all participants were followed up by trained local health staff using telephone. The first interview was conducted within 3 months after baseline examination to track their pregnancy status and record their last menstrual period (LMP), lifestyle, toxicants exposure and dietary habit during early pregnancy as appropriate. If the participants did not get pregnant at the first interview, repeated inquiries were conducted subsequently within the next 3 months until 1 year after baseline examination. Participants who had become pregnant were contacted for pregnancy outcomes within 1 year after the completion of the first follow-up.
Exposure assessment
Preconception Hb concentration was measured with haematology analysers in accordance with National Guide to Clinical Laboratory Procedures. Elevations of over 1000 m can increase most people’s Hb concentrations(Reference Penaloza and Arias-Stella26). In order to obtain the comparative sea-level value, Hb concentrations of women lived in areas over 1000 ms were adjusted by subtracting the adjustment values at the relevant altitude range from the measured Hb concentrations based on one previous study(27), which was suggested by WHO(28). Detailed adjustments and the distribution of participants according to the altitude are presented in online supplementary material, Supplemental Table S1. Then women were successively classified into 3 groups (anaemia: <110 g/l; normal Hb concentration: 110–149 g/l and high Hb concentration: ≥150 g/l), as well as 7 groups (severe anaemia: <70 g/l; moderate anaemia: 70–99 g/l; mild anaemia: 100–109 g/l; normal Hb concentration: 110–149 g/l; mild high Hb concentration: 150–159 g/l; moderate high Hb concentration: 160–169 g/l and severe high Hb concentration: ≥170 g/l), according to definition of anaemia in China(Reference Ge and Xu29).
Outcome
The primary outcome is SA, defined as fetal death, occurring before the 28th week of gestation.
Covariates
The ages of the woman and her husband were calculated as the difference between the date of birth and the first day of the woman’s LMP; then their ages were categorised as 20–24, 25–29, 30–34, 35–39 and ≥ 40 years. Ethnicity was categorised as Han nationality and others. Higher education was defined as levels of education of senior high school, college or higher. Occupation was categorised as famers and others. History of abortion was defined as history of SA or induced abortion. Parity was defined by the number of live births: no previous live birth (nulliparous) and 1 or more live births (parous).
Preconception body weight and height were measured with participants wearing light, indoor clothes and no shoes. BMI was calculated by dividing the weight in kilogram by the square of the height in meter and then was categorised as underweight (<18·5 kg/m2), normal weight (18·5–23·9 kg/m2), overweight (24·0–27·9 kg/m2) and obesity (≥28·0 kg/m2). Seated blood pressure (BP) was measured in the right arm using an automated BP monitor after participants rested for ≥10 min. Hypertension was defined as systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg or self-reported hypertension. Fasting plasma glucose (FPG) and serum thyroid-stimulating hormone (TSH) were measured. Diabetes was defined as FPG ≥ 7·0 mmol/l or self-reported diabetes. Thyroid dysfunction was defined as TSH level of <0·44 mIU/ml or >3·45 mIU/ml, or a history of thyroid disease.
In NFPCP, cigarette smoking was defined as participants who smoked at least one cigarette per day. In the current study, smoking was defined as smoking before or during early pregnancy. Alcohol drinking was defined as drinking before or during early pregnancy. Toxicants exposure was defined as exposure to radiation, organic solvents, heavy metals or pesticides before or during early pregnancy. Abstain from animal products or vegetables was defined as participants who did not eat meat, eggs or hated eating vegetables before or during early pregnancy. Paternal smoking was defined as husband smoking before or during early pregnancy. Paternal toxicants exposure was defined as the husband’s exposure to radiation, organic solvents, heavy metals or pesticides before pregnancy.
Statistical analysis
Baseline characteristics were presented as mean sd or median (interquartile range, IQR) for continuous variables and number (percentage) for categorical variables. The
![]() ${{\chi ^2}}$
test or Kruskal–Wallis test was used to compare the distributions of baseline characteristics according to different preconception Hb levels.
${{\chi ^2}}$
test or Kruskal–Wallis test was used to compare the distributions of baseline characteristics according to different preconception Hb levels.
Logistic regression models were used to estimate the ORs and 95 % CIs of preconception Hb concentrations with risk of SA. Three models were fitted. Model A was a crude model with only Hb groups. Model B adjusted for maternal sociodemographic characteristics, including age at LMP, ethnicity, education and occupation. In model C, we additionally adjusted for maternal information such as BMI, smoking, alcohol drinking, hypertension, diabetes, thyroid dysfunction, history of abortion, parity, toxicants exposure, abstain from animal products or vegetables and paternal information including age at LMP, smoking and toxicants exposure.
We also examined the dose–response relationship of preconception Hb concentrations and SA by restricted cubic spline (RCS). The RCS with 3, 4 or 5 knots was separately fitted, and the model with the lowest Akaike information criterion was chosen as the best model. Finally, the knots for the RCS were set at the 5th, 25th, 50th, 75th and 95th percentiles of Hb concentrations(Reference Schoenaker, Simon and Chaturvedi30). The non-linearity of the dose response was tested by Wald statistics(Reference Frank31). Furthermore, we tested potential interaction with baseline characteristics. Because of the detection of a non-linear relationship between Hb concentrations and SA, in the stratified analysis, we presented the OR and 95 % CI of SA associated with Hb concentrations and tested P interaction by adding an interaction term (binary factor × continuous Hb) to the regression models using RCS.
To examine the robustness of our findings, we also conducted sensitivity analyses by using original Hb concentrations with or without additional adjustment of residence altitude in the logistic regression models. In order to eliminate the effect of thalassemia, sensitivity analysis was also conducted by excluding participants in areas with high prevalence of thalassemia (Provinces: Guangdong, Guangxi, Hainan, Fujian, Yunnan, Guizhou, Sichuan). The R software (V.3.2.2; https://www.r-project.org/) with the ‘speedglm’ and ‘rms’ packages was used for data analyses. Two-sided P values of less than 0·05 were statistically significant.
Results
Among 3 971 428 participants included in the current analysis, 483 650 (12·18 %) women had abnormal preconception Hb concentration, 290 973 (7·33 %) were anaemia and 192 677 (4·85 %) were with high Hb concentration (Table 1). Table 1 shows that compared to women with normal Hb concentrations, women with abnormal Hb concentrations were more likely to be of minority nationalities, be less educated and have pre-existing diabetes or thyroid dysfunction, abstain from animal products or vegetables before or during early pregnancy.
Table 1 Demographic characteristics of the study participants by preconception Hb concentrations

Anaemia, Hb concentration < 110 g/l; normal Hb concentration, Hb in the range of 110–149 g/l; High Hb concentration, Hb ≥ 150 g/l; SA, spontaneous abortion; LMP, last menstrual period.
* Kruskal–Wallis H test was used to examine the differences of baseline characteristics among Hb groups. Others used
![]() ${\chi ^2}$
test.
${\chi ^2}$
test.
† Multiple comparison with Bonferroni-adjusted P value < 0·05 (anaemia group v. normal Hb group; high Hb group v. normal Hb group).
The median length of time from baseline examination to pregnancy was 1·40 months (IQR: 0·40–3·03). Until December 2017, a total of 101 700 SA events were documented, which accounted for 2·56 % of all participants. The proportion of SA in women who were having anaemia, with normal Hb concentration and high Hb concentration, were 2·25 % (6548), 2·57 % (89 679) and 2·84 % (5473), respectively. The crude and multivariable-adjusted ORs of SA across different Hb groups are presented in Table 2. In the fully adjusted model (Model C), compared with women with Hb of 110–149 g/l, significant and increased risk of SA was observed for women with Hb ≥ 150 g/l (OR, 1·10; 95 % CI, 1·07, 1·13), whereas decreased risk of SA was observed for women with Hb < 110 g/l (OR, 0·85; 95 % CI, 0·82, 0·87). Adjusting for different covariates did not substantially affect the estimates, which were similar for models A, B and C. The effect sizes of all covariates in mode C can be found in online Supplementary material, Supplemental Table S2.
Table 2 Associations between maternal preconception Hb concentrations and risk of spontaneous abortion
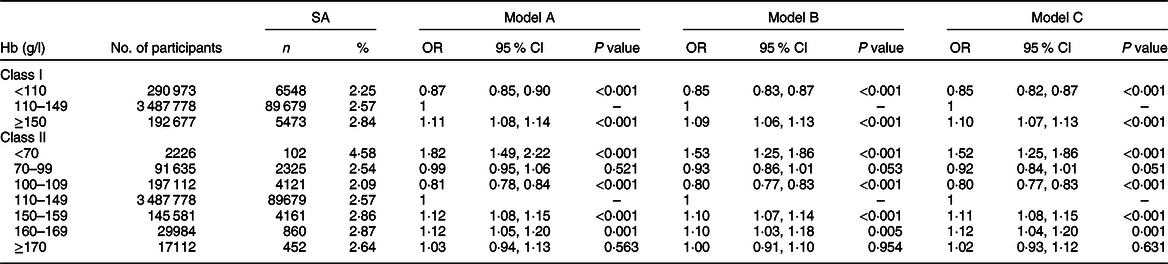
SA, spontaneous abortion.
Model A: ORs were estimated from crude models.
Model B: ORs were adjusted for sociodemographic characteristics of women (age at last menstrual period, education, ethnicity, occupation).
Model C: ORs were additionally adjusted for maternal information (BMI, smoking, alcohol drinking, hypertension, diabetes, thyroid dysfunction, history of abortion, parity, toxicants exposure, abstain from animal products or vegetables) and paternal information (age at last menstrual period, smoking, toxicants exposure).
When anaemia was further subdivided into mild, moderate and severe status, the proportion of SA were 2·09 % (4121), 2·54 % (2325) and 4·58 % (102), respectively. Increased risk of SA was observed for women with severe anaemia (OR, 1·52; 95 % CI, 1·25, 1·86), but negative association was identified with SA for women with mild anaemia (OR, 0·80; 95 % CI, 0·77, 0·83). The proportion of SA in women with mild, moderate and severe high Hb concentration were 2·86 % (4161), 2·87 % (860) and 2·64 % (452), respectively. Mild and moderate high Hb concentration significantly increased the risk of SA with multivariable-adjusted OR of 1·11 (95 % CI, 1·08, 1·15) and 1·12 (95 % CI, 1·04, 1·20). In the sensitivity analyses, the associations between Hb categories and risk of SA did not change appreciably by using original Hb concentration with or without additional adjustment for altitude, or exclusion of participants in areas with high prevalence of thalassemia (online Supplementary material, Supplemental Tables S3 and S4).
Figure 2 showed the crude and multivariable-adjusted dose–response relationships of maternal preconception Hb concentrations with SA. Hb concentrations were associated with SA risk in a U-shaped manner when Hb concentrations were less than 145 g/l, with lowest risk of SA at about 110 g/l to 115 g/l of Hb concentration (P non-linear < 0·001). When Hb concentration was higher than 145 g/l, the association of Hb with SA plateaued in model with adjustment for potential confounding factors.
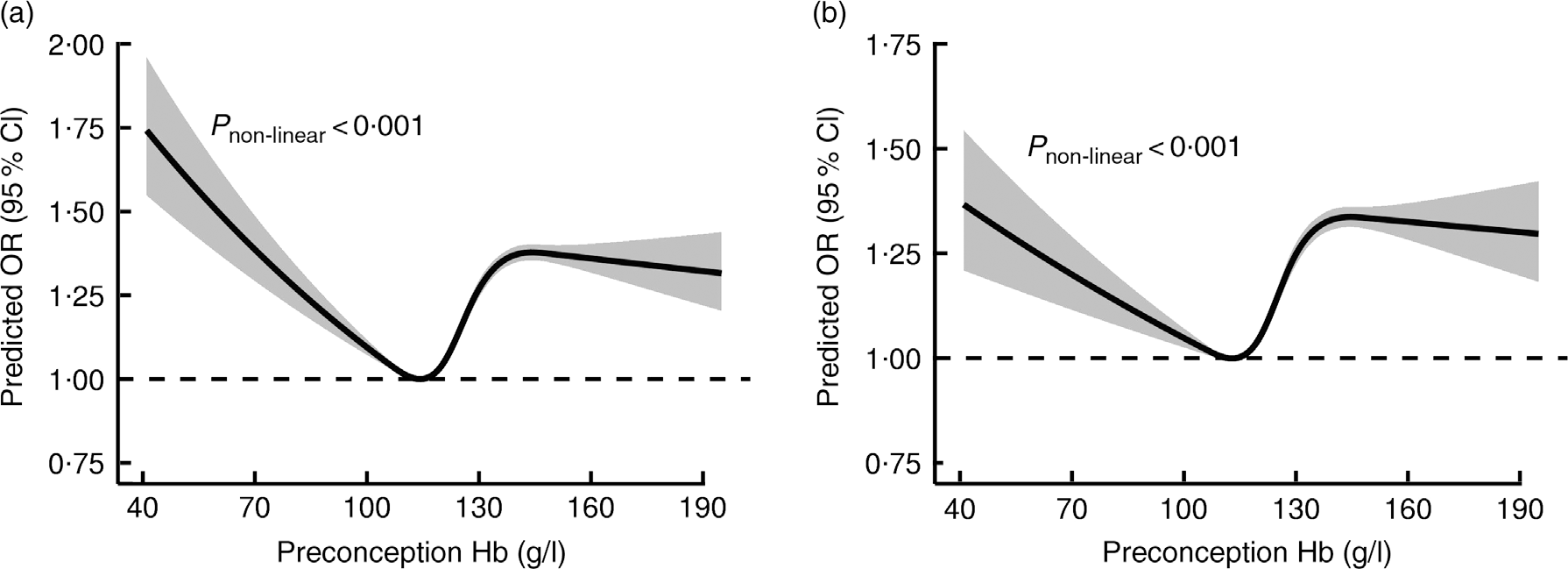
Fig. 2 Dose–response relationship between maternal preconception Hb concentrations and the risk of spontaneous abortion. Graphs show the crude (a) and multivariable-adjusted (b) predicted ORs of associations between maternal preconception Hb concentrations and the risk of spontaneous abortion, respectively. In the graph, black lines and shaded grey areas represent predicted ORs and 95 % CIs, respectively. Study participants with the highest 0·1 % of Hb (3945 women, >195 g/l) were excluded to minimise the potential impact of outliers. Covariates in the multivariable-adjusted model included variables in Model C from Table 2
In the stratified analyses, significant interactions were observed for Hb concentrations with maternal age, paternal age, ethnicity, occupation, diabetes, parity, toxicants exposure, abstain from animal products or vegetables, paternal smoking and altitude, respectively (P interaction < 0·05). The associations between Hb concentrations and SA in women younger than 35 years, whose husbands were younger than 35 years, who were Han nationality, farmers, without diabetes, without toxicants exposure before or during early pregnancy, or living in areas <1000 m, were similar to the results in Table 2 (online Supplementary material, Supplemental Table S5).
Discussion
To the best of our knowledge, this is the first large-scale population-based cohort study to demonstrate the association between preconception Hb concentrations and risk of SA. Our study indicated that severe anaemia and high Hb concentration were associated with an increased risk of SA, independent of other vital risk factors. Furthermore, an approximate U-shaped association for the risk of SA with Hb concentrations was identified when Hb concentrations were less than 145 g/l, above which the association plateaued.
Anaemia has been the most common nutritional disorder among reproductive-aged women across the world, and the worldwide estimated anaemia prevalence for non-pregnant women was about 29 % in 2011 using WHO cutoff points of Hb < 120 g/l(Reference Stevens, Finucane and De-Regil32). This issue is more common in low-income countries, especially in Central and West Africa (48 %) and in South Asia (47 %)(Reference Stevens, Finucane and De-Regil32). Previous studies have shown that maternal anaemia, especially severe anaemia, can be associated with an increased risk of low birth weight(Reference Sekhavat, Davar and Hosseinidezoki33,Reference Steer34) , preterm birth(Reference Zhang, Xu and Yang23,Reference Zhang, Ananth and Li35) and small-for-gestational-age(Reference Kozuki, Lee and Katz36). However, there were only two studies that have assessed the association between maternal anaemia and SA risk. One prospective study including 817 pregnant mothers in Sri Lanka found no association between anaemia and miscarriage, in which Hb concentration was measured at the first antenatal clinic, but gestational age at this time was not specified(Reference Abeysena, Jayawardana and de Seneviratne20). Another recent case–control study included 421 pregnant women that indicated a protective role of mild anaemia against the loss of the fetus, including miscarriage and fetal death(Reference Buzyan21). Similarly, we found mild anaemia was significantly associated with a decreased risk of SA in the current study. The possible reason is that women with known anaemia before pregnancy would actively accept medical treatment and would pay more attention to their Hb levels during pregnancy. It may be easier for women with mild anaemia than those with severe anaemia to improve their anaemia status. Further study is needed regarding the underlying mechanisms. Additionally, even if there was treatment, we still found that maternal preconception severe anaemia (Hb < 70 g/l) was significantly associated with an increased risk of SA, and the incidence of SA was highest among women with severe anaemia, which indicated that pre-pregnancy intervention for anaemia is very necessary. However, there was only 102 women with severe anaemia had SA, indicating the absolute risk is relatively low. This is possibly due to the lower prevalence of severe anaemia in the current study (0·056 %).
The biologic mechanisms underlying a possible association between anaemia and SA may involve the increased susceptibility to infection due to low immunity or the multiple symptoms and signs accompany anaemia, such as headache, fatigue, tachycardia, tachypnoea, which can indirectly affect the pregnancy condition(Reference Williams and Wheby18). Moreover, when maternal Hb concentration decreased to below 70 g/l, pregnant women were at high risk of developing high output cardiac failure and encountered decreased oxygenation of tissues including heart muscle(Reference Williams and Wheby18), which may result in adverse effects on mother and fetus and lead to SA.
Previously, most researchers have paid more attention on anaemia and the possible reasons lie on two aspects: one is that people with high Hb are often neglected in clinical treatment and the other is that the prevalence of high Hb concentration was relatively lower than anaemia(Reference Stevens, Finucane and De-Regil32). However, several studies have already indicated that the increased risk of certain adverse pregnancy outcomes, including preterm birth, low birth weight and maternal morbidities (preeclampsia and gestational diabetes mellitus), can be significantly associated with high Hb concentration before or during pregnancy(Reference Chang, O’Brien and Nathanson14,Reference Abeysena, Jayawardana and de Seneviratne20,Reference Zhang, Xu and Yang23,Reference Phaloprakarn and Tangjitgamol37) . Our study demonstrated that women with preconception Hb ≥ 150 g/l had an increased risk of SA when compared to those with preconception Hb of 110–149 g/l. Our results implied a positive association between maternal high Hb concentration and SA risk; however, evidence of this relationship was quite limited, so further study is warranted to validate our results.
The pathophysiology of the association between maternal high Hb and SA is difficult to elucidate, but it is most likely explained by the known deleterious effects of hyper viscosity. High Hb concentrations can increase blood viscosity and then impair microcirculation and decrease placental blood flow, which could lead to fetal growth impairment directly by compromised nutrient delivery or indirectly by the effect of fetal corticosteroids that are released as a response to chronic hypoxia on the fetus(Reference Yip38,Reference Heilmann39) . More recently, evidence also implied increasing blood viscosity and reducing oxygen levels can lead to oxidative stress, vascular endothelial damage, vasoconstriction and placental ischaemia(Reference Kim-Shapiro, Schechter and Gladwin40). In addition, fetal high Hb concentration can in turn affect maternal circulation and further impairs the placental function(Reference Centlow, Carninci and Nemeth41). All of these changes have been shown to be occurred before the third trimester of pregnancy(Reference Buehler and D’Agnillo42).
In previous studies, U-shaped relationships between maternal Hb concentrations and adverse pregnancy outcomes have been found, but the relations differed by trimester(Reference Chang, O’Brien and Nathanson14,Reference Steer, Alam and Wadsworth43–Reference Gonzales, Steenland and Tapia45) . A recent review showed that adverse outcomes were significantly associated with anaemia when Hb concentration is measured in early pregnancy and associated with high Hb concentration in all three trimesters(Reference Dewey and Oaks46). Our RCS results confirmed that maternal Hb concentrations prior to pregnancy were associated with SA risk in an approximate U-shaped manner, which means an increased risk of SA in relation to either maternal anaemia or high Hb concentration. SA is one kind of a fatal adverse pregnancy outcome for fetus, which is a more severe problem than other outcomes from the fertility perspective(Reference Selevan and Lemasters47). Our findings highlight the importance of monitoring the risk of SA for women with preconception anaemia or high Hb concentration. Therefore, as a simple and effective preconception healthcare strategy, assessing haematological indices in the preconceptional period can detect abnormal Hb status before pregnancy, which gives females at high risk a chance to receive effective intervention and conceive in a better physical condition, to reduce the risk of SA.
As an important nutrient indicator, Hb concentration is highly related with iron levels and nearly half of anaemia cases worldwide were due to iron deficiency(Reference Stevens, Finucane and De-Regil32). Therefore, iron supplementation could be taken into consideration for women with anaemia when they plan to conceive. But several studies suggested that iron supplementation may adversely affect birth outcomes among women without iron deficiency, or with high Hb concentrations(Reference Dewey and Oaks46), so they should use iron supplementation under the guidance of doctors.
As the first large-scale population-based retrospective cohort study, it enables us to divide Hb into multiple groups and ensure enough statistical power of overall analysis. Detailed information regarding lifestyle, reproductive history, diseases history and other important confounding variables, such as periconception toxicants exposure, paternal age and paternal smoking, which have been suggested to be associated with SA(Reference Wang, Yang and Liu10,Reference Slama, Bouyer and Windham48,Reference Kumar49) , were all controlled in the current study. Thus, the relationship between maternal preconception Hb concentrations and SA risk can be well evaluated. In addition, we also found similar results among certain subgroups, which indicated that the relationship between Hb concentrations and SA risk was robust. Furthermore, Hb concentrations before pregnancy within 6 months were used, which minimise the possibility of differential misclassification of the Hb concentrations and confounding by haemodilution with gestational age.
Several limitations of current study should be noted. First, women’s Hb concentrations during pregnancy were not collected, which limited the estimation of the effect of Hb concentrations during pregnancy on SA. Second, other factors associated with Hb concentrations, such as ferritin level or iron supplements intake, were not collected, we cannot distinguish which specific type of anaemia or high Hb concentration is associated with SA. We tried to control the influence of iron supplements by excluding participants who took medication at baseline. However, detailed information about iron supplements of some pregnant women who may take multi-vitamin products, such as Elevit, were not collected, although free prenatal iron supplements were not provided by the Chinese government. Thus, the association between preconception Hb concentrations and SA risk taking iron supplements or ferritin level into consideration need to be further explored. Additionally, we did sensitivity analyses by excluding participants in areas with high prevalence of thalassemia, and the results did not change appreciably. Fourth, the frequency of SA in the current study was lower than other estimates reported in some areas of China (7·9 % to 12·0 %)(Reference Liu and Gao5,Reference Liang and Liu50) . This may have three explanations. Above all, very early SA is often mistaken as a delayed menstrual period, and then this unknown SA event could be wrongly treated as unsuccessful conception, which could lead to underestimate the SA frequency. In addition, participants in the study are from a relatively young and healthy population, with a median age of 25 years, which will lower the SA rate. Further, NFPCP provides preconception health counselling according to the examination results of married couples who wished to conceive within the next six months, which might decrease the frequency of SA compared with couples experiencing unintended pregnancies.
In summary, in this large retrospective cohort study in rural China, we found severe anaemia and high Hb concentration before pregnancy were associated with increased the risk of SA. Women with mild anaemia prior to pregnancy had lower risk of SA, underlying mechanisms need to be further studied. Even the absolute risk of SA is not high due to the lower prevalence of severe anaemia and high Hb levels, our study still indicated that early detection of abnormal Hb status before pregnancy, monitoring the risk for SA, and providing appropriate medical intervention should be considered an important approach for primary prevention of SA, and iron supplementation should be evaluated before pregnancy.
Acknowledgements
Acknowledgements: The authors thank health workers and countless participants throughout 31 provinces in the NFPCP for their great efforts and collaboration. Financial support: This work was supported by National Key Research and Development Program of China (No. 2016YFC1000307), CAMS Innovation Fund for Medical Sciences (No. 2018-I2M-1-004). The sponsors had no role in study design, data analysis, data interpretation, writing of the report or the decision to submit the report for publication. Conflict of interest: None. Authorship: The corresponding author has full access to data in the study and takes responsibility for data integrity and the accuracy of data analysis. Q.X. searched the literature, analysed the data, interpreted the results and drafted the manuscript. L.W., Q.W., H.S., Z.X., Y.Z., D.Y., Y.H, Y. Z., H.Z. and Z.P. collected the data. F.L. revised the manuscript. Y.Y. and X.M. conceived the study, provided overall guidance and revised the manuscript. All authors revised the article for important intellectual content, and each approved the final version to be published. Ethics of human subject participation: The current study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving humans were approved by the Institutional Research Review Board at the National Research Institute for Health and Family Planning. Written informed consent was obtained from all NFPCP participants.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1368980019003811






