Introduction
Drought and salinity are significant plant stressors with major impacts on development and productivity, thus causing serious agricultural yield losses (Tester and Langridge, Reference Tester and Langridge2010; Agarwal et al., Reference Agarwal, Shukla, Gupta and Jha2013). Among these adverse conditions, the increased salinity of arable lands is expected to have a devastating global effect, resulting in up to 50% land loss by the middle of the 21st century (Roychoudhury et al., Reference Roychoudhury, Paul and Basu2013). Similarly, the problem of drought is also quite pervasive and economically damaging (Shao et al., Reference Shao, Chu, Jaleel, Manivannan, Panneerselvam and Shao2009).
The susceptibility of plants to water stress varies, depending on the stress intensity, other accompanying stress factors, plant species and their developmental stages. Drought stress is not only the consequence of a lack of water in the soil but may also be induced by other deleterious environmental conditions, such as salinity, which compromise the plant's ability to take up and to translocate water (Demirevska et al., Reference Demirevska, Zasheva, Dimitrov, Simova-Stoilova, Stamenova and Feller2009). Indeed, the high concentrations of salts in the soil make it harder for roots to extract water, and high concentrations of salts within the plant can be toxic. Salts on the outside of roots have an immediate effect on cell growth and associated metabolism, although toxic concentrations of salts take time to accumulate inside plants before these concentrations affect plant physiology (Munns and Tester, Reference Munns and Tester2008; Geilfus et al., Reference Geilfus, Zörb, Neuhaus, Hansen, Lüthen and Mühling2011). Glycophytic plants, such as the majority of the common crops, are not salt tolerant and are damaged fairly easily by high salinity (Koyro et al., Reference Koyro, Lieth, Said, Lieth, Garcia Sucre and Herzog2008). On the other hand, the halophytes are plants that are able to grow in the presence of high salt concentrations that generate a low water potential of the soil. The main traits of these species are: (1) the ability to tolerate high salinity without losing viability while lying in the soil (usually called secondary seed dormancy); (2) the ability to germinate under high salinity (germination stage); and (3) the ability to complete their life cycle at high salinity (vegetative and reproductive stages) (Khan and Gul, Reference Khan, Gul, Liu and Liu2002). Halophytes can be divided into three categories (Khan, Reference Khan, Lieth, Moschenko, Lohman, Koyro and Hamdy1999): marginally tolerant (able to germinate in NaCl concentrations of up to 125 mM NaCl), moderately tolerant (up to 500 mM NaCl) and highly tolerant (up to 800 mM NaCl or higher). However, the categorization of a plant species as halophytic has proven problematic because the definition involves drawing an arbitrary line in a continuum of tolerance, and also because it involves considerations about the type of salt that is to be tolerated and, finally, how to test this tolerance. However, in both glycophytic and halophytic plants the primary effects of salt stress are caused by the presence of ions in the rhizosphere, limiting water extraction and reducing plant growth, while the secondary effects are caused by a disequilibrium in ion uptake, resulting in enzyme inactivation, nutrient starvation, ionic toxicity and oxidative stress in tissues (Nazar et al., Reference Nazar, Iqbal, Syeed and Khan2011; Khan et al., Reference Khan, Syed, Nazar, Anjum, Khan, Nazar, Iqbal and Anjum2012).
Seed germination is an ontogenetic phase that begins with water uptake by the seed (imbibition) and ends with the start of elongation by the embryonic axis, usually the radicle. When the radicle has grown out of the covering seed layers, the process of seed germination is completed (Hermann et al., Reference Hermann, Meinhard, Dobrev, Linkies, Pesek and Hess2007). It has been demonstrated that seed germination is the most critical phase in plant life and it is greatly influenced by environmental conditions, such as drought and salinity (El-Keblawy and Al-Rawai, Reference El-Keblawy and Al-Rawai2005; Ghavami and Ramin, Reference Ghavami and Ramin2007). Thus, seeds germinating in dry or salty soils have to face serious difficulties. The threshold salinity for a significant reduction in germination varies between species. Some reports interpret the effects of salinity on germination of seeds of different plant species in terms of osmotic potential (Egan et al., Reference Egan, Ungar and Meekins1997), while others explain the reduced germination by ion toxicity (Al-Karaki et al., Reference Al-Karaki, Hammand and Rusan2001). For example, the marked differences in germination percentages obtained with monosaline and bisaline solutions, polyethylene glycol (PEG) and mannitol, at the same osmotic potential, demonstrated specific ionic effects on seed germination of the halophyte Prosopis strombulifera, in addition to the osmotic effects (Sosa et al., Reference Sosa, Llanes, Reinoso, Reginato and Luna2005), suggesting that ionic effects were observed at lower salt concentrations than osmotic effects. Independently of the osmotic and ionic effects of salinity, seeds of halophytes and glycophytes respond similarly to salinity, delaying their germination.
Plant hormones have been shown to modulate several physiological, biochemical and molecular responses during the life cycle of the plant, under both stress and non-stress conditions (Fatma et al., Reference Fatma, Iqbal, Khan, Masood and Khan2013). The main phytohormones include abscisic acid (ABA), indole-3-acetic acid (IAA), ethylene (ET), gibberellins (GAs), jasmonates (JAs), brassinosteroids (BRs), strigolactones (SLs) and nitric oxide (NO). All of these can positively or adversely affect seed germination. Recently, updates on the roles of ABA (Gurmani et al., Reference Gurmani, Bano, Ullah, Khan, Jahangir and Flowers2013), ET and GAs (Iqbal et al., Reference Iqbal, Masood, Khan, Khan, Nazar, Iqbal and Anjum2013), BRs (Choudhary et al., Reference Choudhary, Yu, Yamaguchi-Shinozaki, Shinozaki and Tran2012), SLs (Van Ha et al., Reference Van Ha, Leyva-González, Osakabe, Tran, Nishiyama, Watanabe, Tanaka, Seki, Yamaguchi, Dong, Yamaguchi-Shinozaki, Shinozaki, Estrella and Phan Tran2013), JAs (Khan and Khan, Reference Khan and Khan2013) and NO (Uchida et al., Reference Uchida, Jagendorf, Hibino and Takabe2002) in plant stress responses have been reported. However, the proposed mechanisms involving phytohormones in seed germination under drought and salt conditions vary widely and constitute the focus of this review.
Effects of phytohormones on seed germination under drought and salinity
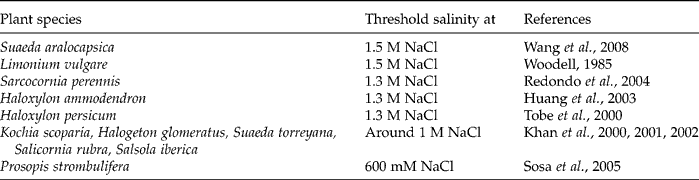
The successful establishment of a plant population is dependent on the adaptive aspects of seed germination. The decrease in germination rate, particularly under drought and salt stress conditions, may be due to the fact that seeds seemingly develop an osmotically enforced ‘dormancy’. Carles et al. (Reference Carles, Bies-Etheve, Aspart, Leon-Kloosterziel, Koornneef, Echeverria and Delseny2002) reported that about 80% of the seeds of abi5-5 and abi5-2, as well as abi4-1, Arabidopsis thaliana mutants germinated on 175 mM NaCl, whereas control seeds did not germinate at this salt concentration. After 2 weeks on 175 mM NaCl, all the mutant seedlings were dead. This is an example showing an adaptive strategy of seeds to prevent germination under harsh environmental conditions, thus ensuring proper establishment of the seedlings (Gill et al., Reference Gill, Sharma, Singh and Bhullar2003). Although seeds of halophytes are quite salt tolerant, seed germination of several halophytes is severely reduced by salinity (Debez et al., Reference Debez, Hamed, Grignon and Abdelly2004; Khan and Gul, Reference Khan, Gul, Khan and Weber2006) and less than 10% may germinate in up to 1.7 M NaCl (Chapman, Reference Chapman1960). Some examples of highly salt-tolerant species are listed in Table 1.
Table 1 Examples of highly salt-tolerant species at the germination stage
Plants have evolved mechanisms to arrest seed germination under stress conditions and to resume it when conditions are favourable. Very early upon imbibition massive transcriptome changes occur, which are regulated by ambient temperature, light conditions and plant hormones. One key mechanism is phytohormone synthesis and metabolism, mainly of GAs and ABA, which have stimulatory and inhibitory effects on seed germination, respectively (Graeber et al., Reference Graeber, Nakabayashi, Miatton, Leubner-Metzger and Soppe2012). It has been shown that exogenous applications of some plant growth regulators can render some benefit to alleviating the adverse effects of drought and salinity (Afzal et al., Reference Afzal, Basra and Iqbal2005; Kaya et al., Reference Kaya, Tuna, Yokas, Ashraf, Ozturk and Athar2009). Therefore, it has been proposed that the unfavourable effects of both drought and salinity on seed germination may include: (1) decreasing the amounts of seed germination stimulants, including GAs; (2) enhancing ABA amounts; and (3) altering membrane permeability and water behaviour in the seed (Lee and Luan, Reference Lee and Luan2012). Notwithstanding, quantitative determinations of endogenous hormone levels during seed germination under stress are scarce for crop plants. Most of what is known about seed germination under abiotic stress as a concerted action of phytohormones has been elucidated in the model plant Arabidopsis, reviewed recently by Golec (Reference Golec2011).
Gibberellins (GAs)
The importance of GAs for seed germination is well known and it was demonstrated by the inability of GA-deficient tomato (gib-1) (Groot et al., Reference Groot, Bruinsma and Karssen1987) and Arabidopsis (ga1-3) (Michaels and Amasino, Reference Michaels and Amasino1999) mutants to germinate without exogenous GA applications. In Arabidopsis and other higher plants, genes encoding GA metabolic enzymes have been isolated, including copalyl diphosphate synthase (CPS), ent-kaurene synthase (KS), ent-kaurene oxidase (KO), ent-kaurenoic acid oxidase (KOA), GA 20-oxidase (GA20ox), GA 3-oxidase (GA3ox) and GA 2-oxidase (GA2ox). The expression levels of these genes have been analysed during seed germination under drought and salinity, and their regulation by both endogenous GA levels and stress conditions has been demonstrated (Ogawa et al., Reference Ogawa, Hanada, Yamauchi, Kuwahara, Kamiya and Yamaguchi2003; Miransari and Smith, Reference Miransari and Smith2009).
Seed priming is a technique that can be applied to improve germination and seedling growth. During priming, the germination process is induced by soaking seeds in water or in solutions containing chemicals such as salts (H.A. Khan et al., Reference Khan, Ayub, Pervez, Bilal, Shamid and Ziaf2009), metals (Mirshekari et al., 2012) or hormones (Nakaune et al., Reference Nakaune, Hanada, Yin, Matsukura, Yamaguchi and Ezura2012), but then halted by drying of the seeds. Primed seeds tend to show better germination and growth even when exposed to stressful conditions. Although the mechanisms involved in the improvement of these parameters by priming are still unclear, it has been suggested that the strategy activates a series of physiological processes that improve plant growth under stressful conditions (Varier et al., Reference Varier, Vari and Dadlani2010), including the induction of antioxidant systems (Eisvand et al., Reference Eisvand, Tavakkol-Afshari, Sharifzadeh, Maddah and Hesamzadeh-Hejazi2010). Hormone pretreatment is a commonly used priming strategy to improve seed germination in stressful conditions (Hu et al., Reference Hu, Zhou, Na, Yang, Nan, Zhang, Li and Bi2013). For example, seeds of rye (Secale montanum) pretreated with gibberellic acid (GA3) increased germination in conditions of water deficit (Ansari et al., Reference Ansari, Azadi, Sharif-Zadeh and Younesi2013). H.A. Khan et al. (Reference Khan, Ayub, Pervez, Bilal, Shamid and Ziaf2009) showed that pretreatment of pepper (Capscum annum L.) with acetylsalicylic acid and salicylic acid resulted in greater uniformity of germination and establishment of seedlings under high salinity. Additionally, ethylene was used to minimize the negative effect of high temperatures on seed germination of lettuce (Lactuca sativa L.) (Nascimento et al., Reference Nascimento, Cantliffe and Huber2004). It has been reported that the major bioactive GA involved in tomato seed priming under salt treatments is GA4. The GA4 level was significantly higher in the NaCl-primed seeds than in control seeds 12 h after sowing and thereafter. Additionally, the peak expression levels of SlEXP4, SlGulB, SlMAN2 and SlXTH4 [genes encoding expansin (EXP), glucanase (GulB), endo-β-mannanase (MAN) and xyloglucan endotransglucosylase hydrolase (XTH), which are enzymes involved in the weakening of the endosperm cap] occurred earlier and were significantly higher in the NaCl-primed seeds (300 mM NaCl) than in the water-primed seeds (Nakaune et al., Reference Nakaune, Hanada, Yin, Matsukura, Yamaguchi and Ezura2012). These results show that the effect of NaCl priming on tomato seeds would be caused by an increase of GA4 levels via GA biosynthetic gene activation and a subsequent increase in the expression of genes related to endosperm cap weakening.
In halophytes, the inhibition of seed germination was caused partly by a strong decline in GA levels during imbibition in saline solution; the exogenous application of GA is known to alleviate efficiently the adverse effect of salinity on germination in some halophytes (Debez et al., Reference Debez, Chaibi and Bouzid2001). In Crithmun maritimum, germination under salinity (100 mM NaCl) was improved by GA3 application, and the application of paclobutrazol, a GA-biosynthesis inhibitor, impaired the germination process (Atia et al., Reference Atia, Debez, Barhoumi, Smaoui and Abdelly2009). The exogenous application of GA was found to be effective in mitigating the NaCl effect on germination of several halophytes, including Zygophyllum simplex, Arthrocnemum indicum L. and Prosopis juliflora (Khan et al., Reference Khan, Gul and Weber2000, Reference Khan, Gul and Weber2002; El-Keblawy et al., Reference El-Keblawy, Al-Ansari and Al-Rawai2005). However, changes in endogenous GA levels and signalling in seed germination under drought and salinity remain unclear. In addition, evidence presented by different studies suggests that regulatory genes involved in the control of ABA and GA levels during imbibition can differ, not only inter- and intraspecifically, but also in response to different environmental factors that affect germination (Rodríguez et al., Reference Rodríguez, Mendiondo, Cantoro, Auge, Luna, Masciarelli and Benech-Arnold2012). Thus, it might be difficult to make generalizations to describe a unique model for germination under stress, but the general tendency seems to be similar to the process of dormancy termination, involving the stimulation of active GA biosynthetic pathways, concomitantly with stimulation of ABA catabolism, such as hydroxylation and conjugation. There are three different ABA hydroxylation pathways that oxidize one of the methyl groups of the ring structure (C-7′, C-8′ and C-9′), and conjugate ABA with glucose to produce ABA glucosyl ester (ABA-GE).
Abscisic acid (ABA)
As described above, ABA regulates seed germination and one of the proposed mechanisms affects the cell cycle. Inhibition of the cell cycle by ABA is related to activation of a residual G1 kinase, which becomes inactivated in the absence of ABA (Sánchez et al., Reference Sánchez, Gurusinghe, Bradford and Vazquez-Ramos2005; Nitsch et al., Reference Nitsch, Oplaat, Feron, Ma, Wolters-Arts, Hedden, Mariani and Vriezen2009). This is the reason for the rapid germination of seeds that are deficient in ABA.
Changes in ABA content in the seed as a result of adverse environmental conditions can influence seed germination (Ali-Rachedi et al., Reference Ali-Rachedi, Bouinot, Wagner, Bonnet, Sotta, Grappin and Jullien2004; Alboresi et al., Reference Alboresi, Gestin, Leydecker, Bedu, Meyer and Truong2006). Andrade et al. (Reference Andrade, Vigliocco, Alemano, Alvarez and Abdala2009) determined ABA and ABA catabolite contents in germinated seeds of two inbred lines of sunflower (drought-sensitive B59 and drought-tolerant B71) growing under irrigation and drought. For drought treatment, the soil was covered with polypropylene sheets until harvest, with no irrigation; and for irrigation treatment, plants were watered when soil moisture fell to 60% of field capacity. ABA levels in seeds decreased after germination in both B71 and B59 lines, although the ABA decrease in B71 seeds from irrigated plants was only moderate. Germination triggered a substantial increase in the ABA catabolite ABA-GE in seeds under all treatments. It was proposed that the water status during growth of the mother plant affected ABA biosynthesis and metabolism in mature and germinated seeds.
Accordingly, Chono et al. (Reference Chono, Honda, Shinoda, Kushiro, Kamiya, Nambara, Kawakami, Kaneko and Watanabe2006) found that environmental conditions of the mother plant during seed development, such as humidity, salinity and temperature, affected the expression of HvNCED1, a key gene for ABA biosynthesis, and HvCYP707A1, an ABA deactivation gene, in barley grains, resulting in different ABA contents. ABA contents were evaluated in tomato seeds soaked in 300 mM NaCl (NaCl priming) as compared to distilled water (hydro-priming). For both priming treatments, the ABA content of the seeds increased during treatment but rapidly decreased after sowing. Both during and after the priming treatments, ABA contents of the hydro-primed seeds and NaCl-primed seeds were not significantly different. Thus, the presence of salt did not influence the ABA levels in these seeds, and they remained high during the priming period (Nakaune et al., Reference Nakaune, Hanada, Yin, Matsukura, Yamaguchi and Ezura2012). These results are in agreement with the suggestion that salt-induced endogenous ABA is due to water deficit rather than specific salt effects (Zhang et al., Reference Zhang, Garreton and Chua2005).
Positive effects on seed germination following the exogenous supply of fluridone, an ABA-biosynthesis inhibitor, have been reported in some halophytes. This is in agreement with the concept that dormancy is an active process tightly related to de novo ABA synthesis. If suppression of germination requires de novo ABA synthesis, as described for dormant seeds, the residual ABA produced during embryogenesis may be irrelevant (Finkelstein et al., Reference Finkelstein, Gampala and Rock2002). Non-dormant seeds complete germination once imbibed or may enter secondary dormancy if the environmental conditions are unfavourable for germination, which is also related to de novo ABA synthesis. However, in other species, ABA is high at maturity, and increased germinability is correlated with the decline of endogenous ABA contents. ABA hydroxylation at the C-8 position is considered to be the predominant ABA inactivation pathway and it appears to be critical in regulating seed dormancy release (Nambara and Marion-Poll, Reference Nambara and Marion-Poll2005; Gubler et al., Reference Gubler, Hughes, Waterhouse and Jacobsen2008). Nevertheless, the knowledge and understanding of changes in ABA content and its role in regulating seed germination specifically under drought and salinity are still inconclusive, maybe because these two stresses cause an osmotically induced endodormancy that may overlap with the natural dormancy of the species, making it difficult to distinguish the actual causes of hormonal variations.
Auxins
Contrary to the roles of GAs and ABA in seed germination, auxin is not an essential hormone for seed germination (Chiwocha et al., Reference Chiwocha, Cutler, Abrams, Ambrose and Yang2005). However, according to the analyses regarding the expression of auxin-related genes, auxin is present in the radicle tip during and following seed germination. In addition, microRNA160 inhibits auxin-responsive factor (ARF10) during seed germination, allowing the seed to germinate. Such a controlling process is also necessary for stages related to post-germinative growth. Although indole-3-acetic acid (IAA) by itself may not be important for seed germination, its interactions and cross-talk with other phytohormones may influence this process (Harberd, Reference Harberd2003; Chiwocha et al., Reference Chiwocha, Cutler, Abrams, Ambrose and Yang2005). Thus, changes in endogenous IAA levels during seed germination were reported in lettuce and in Arabidopsis wild type and its etr mutant (Chiwocha et al., Reference Chiwocha, Cutler, Abrams, Ambrose and Yang2005). In germinating pea seeds, IAA affected seed germination through the regulation of glyoxalase I activity, resulting in higher rates of cell growth and development (Hentrich et al., Reference Hentrich, Bottcher, Duchting, Cheng, Zhao, Berkowitz, Masle, Medina and Pollmann2013). Seo et al. (Reference Seo, Nambara, Choi and Yamaguchi2009) reported that an Arabidopsis R2R3-type MYB transcription factor, MYB96, regulates drought stress responses by integrating ABA and auxin signals in seed germination. During both germination and seedling growth, endogenous IAA levels were unaltered in the myb96-ox mutant, which would be due to rigorous control of auxin homeostasis under stress conditions (Dombrecht et al., Reference Dombrecht, Xue, Sprague, Kirkegaard, Ross, Reis, Fitt, Sewelan, Schenk, Manners and Kazan2007; Park et al., Reference Park, Park, Kim, Staswick, Jeon, Yun, Kim, Kim, Lee and Park2007). Similarly, Jung and Park (Reference Jung and Park2011) demonstrated that seed germination in Arabidopsis is suppressed by auxin under high salinity (150 mM NaCl). These authors generated transgenic plants overexpressing the YUCCA3 (YUC3) gene, which encodes an auxin biosynthetic enzyme (Zhao et al., Reference Zhao, Christensen, Fankhauser, Cashman, Cohen, Weigel and Chory2001). Seed germination of the YUC3-overexpressing plants was more sensitive to high salinity than that of the wild type. Moreover, a membrane-bound NAC transcription factor, NTM2, isolated from Arabidopsis seeds (Kim et al., Reference Kim, Park, Hwang, Lee, Oh and Kim2006; Seo et al., Reference Seo, Nambara, Choi and Yamaguchi2009; Jung and Park, Reference Jung and Park2011) mediates the signalling cross-talk between auxin and salt stress via the IAA30 gene during seed germination. The deficient ntm2-1 mutant exhibited enhanced resistance to high salinity at germination. However, salt resistance was reduced in the ntm2-1 mutant overexpressing the IAA30 gene, which was induced by high salinity in an NTM2-dependent manner. Exogenous auxin further suppressed the reduced germination rate of control seeds under high salinity. In contrast, the auxin effects disappeared in the ntm2-1 mutant. These observations indicate that NTM2 is a molecular link that incorporates the auxin signal into a salt stress signal during seed germination. Other researchers also reported that pre-sowing wheat seeds with plant growth regulators such as IAA alleviated the growth-inhibiting effect of salt stress (Afzal et al., Reference Afzal, Basra and Iqbal2005). In wheat, seed germination decreased with increasing salinity level, while the adverse effect of salinity was alleviated by treatment of seeds with IAA or 1-naphthaleneacetic acid (NAA) (Gulnaz et al., Reference Gulnaz, Iqbal, Farooq and Azam1999). Then, as for GAs, it might be thought that applications of IAA, both plant growth promoters associated with plant development, would temporarily force the machinery of the emerging seedling to enhance growth in an apparent alleviation of salinity adverse effects. In contrast, when stress treatments are applied to germinating seeds without exogenous application of plant growth promoters, their endogenous levels decrease to arrest the process, to avoid high energy costs under unfavourable conditions.
Ethylene (ET)
There are different opinions regarding the role of ET in seed germination. Some studies have proposed that ET is produced as a result of seed germination, while others suggested that this hormone is necessary for seed germination in a wide range of species (Gianinetti et al., Reference Gianinetti, Laarhoven, Persijn, Harren and Petruzzelli2007; Matilla and Matilla-Vazquez, Reference Matilla and Matilla-Vazquez2008). Mayak et al. (Reference Mayak, Tirosh and Glick2004) reported that ET production was enhanced in tomato seeds exposed to drought and salinity, and Matilla (Reference Matilla2000) reported that ET, ethephon (an ethylene-releasing compound) or ACC (1-aminocyclopropane-1-carboxylic acid, an ethylene precursor) stimulated seed germination under non-optimal conditions. These results could be explained by the effect of ET in overcoming the inhibitory effect of ABA in seed germination (Kucera et al., Reference Kucera, Cohn and Leubner-Metzger2005). Accordingly, Wu et al. (Reference Wu, Raza, Fan, Sun, Bao, Liu, Huang, Mao, Shen and Miao2008) demonstrated that the expression of JERF3, a subfamily of ethylene responsive factor (ERF) proteins, increased concomitantly with germination in tobacco seeds under osmotic stress induced by mannitol (0.2 M). This study confirmed that JERF3 expression in tobacco plants enhanced adaptation to osmotic stress, which suggests a possible role of ET in germination under drought stress. Similarly, alleviation of the detrimental effects of salinity on seed germination by ET has been shown in numerous species (Mohammed, Reference Mohammed2007; M.A. Khan et al., Reference Khan, Ayub, Pervez, Bilal, Shamid and Ziaf2009). Bialecka and Kępczyński (Reference Białecka and Kępczyński2009) clearly demonstrated the ability of ethephon (0.1 mM) to alleviate the inhibition of Amaranthus caudatus seed germination under salinity (50–125 mM NaCl).
Recently, Silva et al. (Reference Silva, Medina, Barros and Ribeiro2014) developed interesting experiments to analyse the role of endogenous ET in seed germination. They employed seeds of Stylosanthes humilis, S. capitata and S. guianensis to examine the mode of action of salinity stress on the germination process and the importance of linkages between ET biosynthesis and embryo hypocotyl–radicle axis growth for sustaining germination of seeds under salt stress conditions. This study demonstrated that the three species differ in their ET production and germination responses to the imposed salinity stress. These differential responses involved ET as an aspecific modulator against salt toxicity during germination. Thus, the higher ET biosynthesis in S. guianensis seeds than that of S. humilis and S. capitata can effectively mitigate against salt stress evoked by accumulation of high levels of Na in its seed tissues. These results show that NaCl inhibits seed germination through inhibition of ET biosynthesis. The differences in NaCl stress tolerance shown by the three species may be explained by their different abilities to synthesize ACC, associated with corresponding ET biosynthesis. Therefore, this study highlights the exceptional flexibility of the ET pathway in response to salinity stress.
Several reports suggest that ET acts on ABA metabolism to reduce ABA levels and to improve seed germination under saline conditions. Indeed, the mutations that reduce ET sensitivity (e.g. etr1, ein2 and ein6) result in an increase in ABA sensitivity, while increased ET sensitivity in ctr1 and eto1 reduces ABA sensitivity (Kucera et al., Reference Kucera, Cohn and Leubner-Metzger2005; Linkies et al., Reference Linkies, Müller, Morris, Tureckova, Wenk, Cadman, Corbineau, Strnad, Lynn, Finch-Savage and Leubner-Metzger2009; Subbiah and Reddy, Reference Subbiah and Reddy2010). Moreover, genetic evidence indicates that ET and ABA may also act in parallel, since double mutants obtained by crossing ET mutants (ctr1, ein1, ein3 and ein6) with the aba2 mutant exhibited phenotypes resulting from both ABA deficiency and altered ET sensitivity (Cheng et al., Reference Cheng, Chiang, Hwang and Lin2009). Nevertheless, treatments with exogenous ET-releasing compounds or ACC did not affect ABA levels or expression of genes involved in ABA biosynthesis in Lepidium sativum (Linkies et al., Reference Linkies, Müller, Morris, Tureckova, Wenk, Cadman, Corbineau, Strnad, Lynn, Finch-Savage and Leubner-Metzger2009) and sugar beet (Hermann et al., Reference Hermann, Meinhard, Dobrev, Linkies, Pesek and Hess2007). Recently, Wilson et al. (Reference Wilson, Kim, Bakshi and Binder2014) reported that, in contrast to ET which can act either as a negative or a positive regulator of salt tolerance, ET signalling positively regulates salt tolerance. Indeed, ET receptor mutants such as etr1 and ein4 show increased salt sensitivity (Cao et al., Reference Cao, Costa, Biderre-Petit, Kbhaya, Dey, Perez, McCarty, Gutierrez-Marcos and Becraft2007). Somewhat unexpectedly, however, the two ET receptors ETR1 and ETR2 differentially affect salt tolerance during seed germination. Mutations in ETR1 and EIN4 accelerate, whereas those in ETR2 delay, germination under salt stress. Interestingly, such contrasting effects of different ET receptors on salt tolerance do not seem to be due the effects of these mutations on ET production or sensitivity. Instead, it appears that, under salt stress, ETR1 and EIN4 stimulate ABA biosynthesis to inhibit germination. ETR2, by suppressing ETR1 and EIN4, reduces ET levels and improves germination under salt stress (Wilson et al., Reference Wilson, Kim, Bakshi and Binder2014).
Jasmonic acids (JAs)
JAs have been reported to have various effects on seed dormancy and germination. It has been shown that JA can inhibit seed germination in several species, such as Solanum lycopersicum, Brassica napus, Linum usitatissimum, Lupinus luteus and Zea mays (Wilen et al., Reference Wilen, van Rooijen, Pearce, Pharis, Holbrook and Moloney1991; Norastehnia et al., Reference Norastehnia, Sajedi and Nojavan-Asghari2007; Miersch et al., Reference Miersch, Neumerkel, Dippe, Stenzel and Wasternack2008; Oh et al., 2009; Zalewski et al., Reference Zalewski, Nitkiewicz, Lahuta, Głowacka, Socha and Amarowicz2010). Also, methyl jasmonate (MeJA), a volatile JA metabolite, inhibits germination of seeds of cocklebur, lettuce, sunflower, Amaranthus, native tobacco (Nicotiana attenuata), oat, wheat, rapeseed and flax (Daletskaya and Sembdner, Reference Daletskaya and Sembdner1989; Preston et al., Reference Preston, Tatematsu, Kanno, Hobo, Kimura, Jikumaru, Yano, Kamiya and Nambara2009), but it enhances germination of a number of dormant seeds, including apple, pear and two species of Acer (Daletskaya and Sembdner, 1989; Berestetzky et al., Reference Berestetzky, Dathe, Daletskaya, Musatenko and Sembdner1991; Ranjan and Lewak, Reference Ranjan and Lewak1992; Jarvis and Davies, Reference Jarvis and Davies1997; Yildiz et al., Reference Yildiz, Yazici and Muradoglu2007, Reference Yildiz, Cenkci and Kargioglu2008). Some studies, based on crossing several Arabidopsis mutants impaired at several steps during β-oxidation, showed that, in fact, 12-oxo phytodienoic acid (OPDA), and not JA, has the stronger effect in inhibiting seed germination, and they found that OPDA was around ten times more efficient than JA. These studies suggest a synergistic effect of OPDA and ABA on the germination inhibition of A. thaliana seeds (Dave et al., Reference Dave, Hernández, He, Andriotis, Vaistij, Larson and Graham2011; Wasternack, Reference Wasternack2014). Taken together, this background of studies demonstrates the involvement of JAs in seed germination under optimal conditions. However, knowledge about how germination under drought and salinity is regulated by JAs is still incomplete. In spite of this, Andrade et al. (Reference Andrade, Vigliocco, Alemano, Miersch, Botella and Abdala2005) reported an accumulation of JAs during germination in tomato seeds of cv. Moneymaker and the hypersensitive mutants tss1, tss2 and tos1 under saline conditions. In addition, Vigliocco et al. (Reference Vigliocco, Alemano, Miersch, Alvarez and Abdala2007) observed that OPDA content increased after imbibition for 72 h in seeds from sunflower plants grown under both irrigation and drought. Another JA metabolite, 12-hydroxyjasmonic acid (12-OH-JA), decreased in the pericarp while the embryonic axis and cotyledons showed an increase in the level of this compound. These overall results show the existence of an ‘oxylipin signature’ among dry seeds and imbibed seeds, reflecting the JA changes during germination.
Brassinosteroids (BRs)
BRs can overcome the adverse effect of abiotic stress on seed germination in almost all endospermic seeds, but they do not have any effect on germination of some non-endospermic seeds (Leubner-Metzger, Reference Leubner-Metzger2001). Thus, the addition of BRs during drought treatment improved seed germination of radish (Raphanus sativus) (Mahesh et al., Reference Mahesh, Parshavaneni, Ramakrishna and Rao2013). This phytohormone also promoted seed germination by enhancing the growth potential of tobacco embryos (Leubner-Metzger, Reference Leubner-Metzger2001) and overcoming the ABA inhibition of germination in Arabidopsis (Steber and McCourt, Reference Steber and McCourt2001). In Arabidopsis, the protein GPA1 (G protein alpha 1) has been shown to be involved in BR signalling. This gene controls BR-mediated elongation of the hypocotyl and BR-controlled GA sensitivity of seed germination (Ullah et al., Reference Ullah, Chen, Young, Im, Sussman and Jones2001, Reference Ullah, Chen, Wang and Jones2002; Gao et al., Reference Gao, Liu, Bi, Zhang, Cheng, Chen and Zhang2008). In wild-type Arabidopsis, BR can rescue the inhibition of seed germination by GA deficiency. In contrast, both gpa1 and BR-insensitive bri1 mutants failed to rescue seed germination. The gpa1mutant was less sensitive to BR than the wild-type plant in the BR-mediated control of hypocotyl and root elongation. Accordingly, some studies have demonstrated an interaction between GAs and BRs on seed germination (Steber and McCourt, Reference Steber and McCourt2001; Kasahara et al., Reference Kasahara, Hanada, Kuzuyama, Takagi, Kamiya and Yamaguchi2002). Indeed, exogenous GAs, brassinolide (BL) (1 mg l− 1) and fluridone, an inhibitor of carotenoid biosynthesis, significantly increased the broomrape seed response to a germination stimulant (GR24, 10− 6M) even when seeds were first hydro-primed. Exogenous GA3 and BL could restore the germination of Orobanche spp. seeds (Song et al., Reference Song, Steinebrunner, Wang, Stout and Roux2006), perhaps by breaking the secondary dormancy induced by water stress. However, the hierarchy of the role of BRs in the regulation of germination is not yet clear. To determine the influence of 24-epibrassinolide (24-EBL) on salt-stress-induced inhibition of Brassica napus, seeds were allowed to germinate on a nutrient medium containing 1 or 2 μM 24-EBL and different concentrations of NaCl (Kagale et al., Reference Kagale, Divi, Krochko, Keller and Krishna2007). The presence of 24-EBL (2 μM) in the medium considerably reduced the inhibitory effect of high salt on seed germination, as evidenced by an increase in germination and early seedling growth. Seeds of chickpea (Cicer arietinum L. cv. KPG-59) imbibed in an aqueous solution of 10− 10 or 10− 8M 28-homobrassinolide (28-HBL) and NaCl (1 or 10 mM) were evaluated (Ali et al., Reference Ali, Hahn and Paek2007). The seedlings resulting from the seeds soaked in 28-HBL (10− 8M) possessed higher leaf nitrate reductase and carbonic anhydrase activities, more dry mass, higher nodule number and greater nodule fresh and dry masses, compared with those resulting from water-soaked seeds. These values declined significantly in seedlings raised from the seeds soaked in NaCl. This effect was overcome if NaCl treatment was given before or after HBL treatment. Overall, these studies show that BRs improve seed germination under adverse conditions through interaction with other plant hormones. For example, Divi et al. (Reference Divi, Rahman and Krishna2010) reported that 24-EBL increased salt tolerance of Arabidopsis mutants that are either deficient in ABA or insensitive to ABA, ET, JA and salicylic acid (SA). The positive impact of 24-EBL on mutants compared to the wild type was evident in all mutants studied, with the exception of the SA-insensitive npr1-1 mutant. 24-EBL could rescue the ET-insensitive ein2 mutant from its hypersensitivity to salt-stress-induced inhibition of seed germination. However, it is not known whether changes in endogenous BR levels are normally involved in mediating seed germination under stressful conditions.
Strigolactones (SLs)
A small class of carotenoid-derived compounds, the SLs, was first characterized more than 45 years ago as seed germination stimulants in root-parasitic plants, such as Striga, Orobanche and Phelipanche species (Xie et al., Reference Xie, Yoneyama and Yoneyama2010; Ruyter-Spira et al., Reference Ruyter-Spira, Al-Babili, van derKrol and Bouwmeester2013). Van Ha et al. (Reference Van Ha, Leyva-González, Osakabe, Tran, Nishiyama, Watanabe, Tanaka, Seki, Yamaguchi, Dong, Yamaguchi-Shinozaki, Shinozaki, Estrella and Phan Tran2013) provided direct evidence that SLs positively regulate drought and high salinity responses in Arabidopsis. Both SL-deficient and SL-response max (more axillary growth) mutants exhibited hypersensitivity to salt stress. These mutants exhibited a lower sensitivity to various concentrations of ABA compared with wild-type seeds during germination and post-germination events. These results indicate that the cross-talk between SLs and ABA plays an important role in integrating stress signals, although changes in endogenous SL levels and their role in seed germination under drought and salinity remain as yet unknown.
Nitric oxide (NO)
NO is a signal molecule involved in the control and regulation of a variety of plant responses to environmental stresses, in almost all stages of plant development (Zhao et al., Reference Zhao, Tian and Zhang2007). Some reports describing the stimulation of seed germination of various plant species by treatment with NO-releasing chemicals have been published. For example, NO was reported to stimulate seed germination in lettuce (Lactuca sativa L. cv. Grand Rapids) (Beligni and Lamattina, Reference Beligni and Lamattina2000), confirming its significant role in the termination of dormancy and the enhancement of seed germination (Bethke et al., Reference Bethke, Libourel and Jones2006; Gniazdowska et al., Reference Gniazdowska, Krasuska and Debska2010; Liu et al., Reference Liu, Mehdi, Topping, Tarkowski and Lindsey2010). In the breaking of Arabidopsis seed dormancy there was a decrease in ABA content, which was induced by a rapid accumulation of NO (Liu et al., Reference Liu, Song, Chen and Yu2009). These authors demonstrated that during imbibition there was a rapid release of NO to the endosperm layer, which preceded a rise in activity of ABA catabolism, which allowed subsequent seed germination. Some studies suggested that pretreatment of seeds with NO may be used as a part of the procedure leading to seed quality improvement, because NO can decrease sensitivity of seeds to various abiotic stresses. Thus, sodium nitroprusside (SNP), commonly used as a NO-donor, was shown to alleviate the effect of salt stress during wheat germination (Duan et al., Reference Duan, Yang, Lu, Korpelainen, Berninger and Li2007; Zheng et al., Reference Zheng, Jiang, Liu, Dai, Liu, Jing and Cao2009). Likewise, Arabidopsis mutants (atnoa and nia1nia2) with reduced NO accumulation showed an enhanced sensitivity to salt and osmotic stress when their seeds were germinated in medium supplemented with 125 mM NaCl or 250 mM mannitol (Lozano-Juste and Leon, Reference Lozano-Juste and Leon2010). NO exerts its regulatory activity in the stimulation of seed germination under drought and saline conditions in tight coordination with other molecules, such as reactive oxygen species (ROS) (Wang et al., Reference Wang, Liang, Wan, Wang and Bi2009). For example, in cucumber seeds (Cucumis sativus L.) NO-induced resistance of salt stress was associated with activation of superoxide dismutase (SOD) and catalase (CAT) (Fan et al., Reference Fan, Yan, Zhang and Xu2013). In cells of barley aleurone layers, a delay of programmed cell death by NO was demonstrated, which correlated with the stimulation of antioxidant enzyme activities (Beligni et al., Reference Beligni, Fath, Bethke, Lamattina and Jones2002). Recently, Krasuska et al. (Reference Krasuska, Ciacka, Debska, Bogatek and Gniazdowska2014) were able to create a model of the ‘nitrosative door’, describing direct links between NO, ROS and phytohormones in the regulation of seed dormancy and germination. In this model appropriate levels of NO (the ‘nitrosative door’) are required for dormancy alleviation and stimulation of seed germination. Below this level, the amount of NO is too low to promote germination, while overaccumulation/overproduction of NO leads to damage of cellular components that prevent or delay germination. Therefore, NO acts as a signalling molecule necessary to induce metabolic changes responsible for seed transition from dormancy to germination. Although this model was proposed for germination under optimal conditions, it may possibly be applied to seeds exposed to adverse environmental conditions.
Conclusions and perspectives
By affecting the hormonal balance in the seed, environmental parameters including salinity, acidity, temperature and light influence seed germination. A model integrating the different hormone levels in germinating and non-germinating seeds under conditions of drought and salinity is depicted in Fig. 1. GAs, ET, SLs and BRs induce seed germination by rupturing testa and endosperm, while antagonistically interacting with the inhibitory effects of ABA and, to a minor extent, JAs on seed germination. NO is an important signalling molecule acting by itself or as a second messenger, enhancing germination and reducing the expression of dormancy.
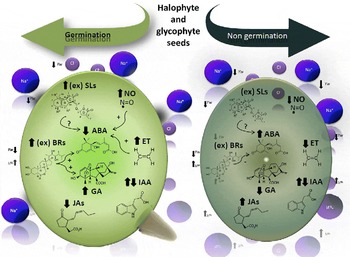
Figure 1 The model shows the endogenous level of each phytohormone involved in glycophytic and halophytic seed germination or non-germination under drought (represented by Yw) and salinity (represented by Na+ and Cl−). Increases in endogenous hormone levels are shown by upward arrows ( ↑ ) and decreases are shown by downward arrows ( ↓ ); ex: exogenous applications of hormones. Interactions among hormones: positive interactions are indicated by (+) and negative by ( − ), whereas unknown interactions are indicated by (?). ABA, abscisic acid; IAA, indole-3-acetic acid; ET, ethylene; GAs, gibberellins; JAs, jasmonates; BRs, brassinosteroids; SLs, strigolactones; NO, nitric oxide.
The increasing amount of experimental data reporting discoveries of new endogenous regulators and related gene expression will further broaden our insight into the importance of endogenous hormonal profiles mediating seed germination under conditions of drought and salt stress. Although some contradictory data reflect the variability in hormonal responses to both types of stress, it is clear that the seed hormonal profiles share common components as sets of specific regulatory factors that are likely working in different parts of the seed. In recent decades, several components of the mechanisms underlying the cross-talk among different phyto-hormone signalling pathways have been identified, leading to the elucidation of partial or entire cross-talk cascades. By now, it is imperative to address questions such as why, in GA-deficient mutants, ET, BRs and SLs can act similarly to GAs, as the seeds are able to germinate in such a situation. Use of mutants is one of the most interesting ways to determine the role of each plant hormone in seed germination. With this focus, it has been concluded recently that hydrogen peroxide mediates the up-regulation of ABA catabolism, probably through an NO signal, and also promotes GA biosynthesis. High concentrations of ABA inhibit GA biosynthesis, but a balance of these two hormones may control the dormancy and germination of Arabidopsis seeds under non-stressed conditions. Current research combining genetic tools and recent technologies should help to clarify the roles of phytohormones in seed germination under conditions of drought and salinity more accurately, and to elucidate the complex interactions between alterations in endogenous hormonal levels underlying the physiological responses of germinating seeds to both stresses.
Conflicts of interest
None.




