Electroconvulsive therapy (ECT) is widely considered to be the most effective treatment for severe major depression. However, there continues to be controversy in the field about optimal methods for administering the treatment. In particular, electrode placement, that is, the anatomic location of the stimulus electrodes on the individual's scalp, has been the subject of debate for more than 60 years. Reference Friedman1–Reference Fink and Taylor7 This debate centres around the balance of the antidepressant efficacy of the treatment against the cognitive effects it produces. Numerous studies Reference Letemendia, Delva, Rodenburg, Lawson, Inglis and Waldron8–Reference Sackeim, Prudic, Devanand, Kiersky, Fitzsimons and Moody10 and a meta-analysis 11 have concluded that right unilateral ECT is moderately less effective than bitemporal ECT and that it causes fewer cognitive effects. Recently, however, study data suggest that right unilateral electrode placement must be delivered at multiples of seizure threshold to be maximally effective. Reference Abrams6,Reference Sackeim, Prudic, Devanand, Kiersky, Fitzsimons and Moody10,Reference McCall, Reboussin, Weiner and Sackeim12 Thus, much of the literature prior to 2000 contains results that are biased against efficacy in right unilateral placement. Reference Abrams6 A novel placement, bifrontal, has recently gained popularity in clinical practice because it is reported to be equally efficacious to bitemporal placement, but with fewer cognitive effects. Reference Letemendia, Delva, Rodenburg, Lawson, Inglis and Waldron8,Reference Bailine, Rifkin, Kayne, Selzer, Vital-Herne and Blieka13 The significance of the cognitive effects of ECT as a basis for electrode selection remains highly controversial. Reference Sackeim14 Some experts contend that these effects are of little importance compared with the often dramatic lifesaving effects of the treatment. Reference Fink15 Yet, cognitive effects are the main impediment to the broader application of ECT. Reference Shorter and Healy16,Reference Prudic17 To our knowledge, no prior study has directly compared bitemporal, bifrontal and right unilateral ECT. We carried out a multisite, randomised, clinical trial using modern state-of-the-art ECT techniques and comprehensive masked assessments to address the above issues.
Method
Overview
The participating centres (University of Medicine and Dentistry of New Jersey-New Jersey Medical School, Medical University of South Carolina, The Zucker-Hillside Hospital Northshore-Long Island Jewish Health System, University of Texas Southwestern Medical Center at Dallas and Mayo Clinic) comprise the Consortium for Research in ECT (CORE). Reference Petrides, Fink, Husain, Knapp, Rush and Mueller18,Reference Kellner, Knapp, Petrides, Rummans, Husain and Rasmussen19 This study was a multicentre, National Institute of Mental Health (NIMH)-funded, randomised, double blind, controlled trial carried out from 2001 to 2006 (NCT00069407). A total of 230 people with acute depression, both bipolar and unipolar, were randomly assigned using a permuted block-randomisation scheme to one of three electrode placements during an acute course of ECT: bifrontal at one and a half times seizure threshold, bitemporal at one and a half times seizure threshold, and right unilateral at six times seizure threshold. Participants were treated until they achieved pre-specified remission criteria and then were followed naturalistically for 2 months. A comprehensive neurocognitive battery was performed at baseline, after the fourth ECT, after the last ECT and at 1 week and 2 months after the last ECT. This protocol was reviewed and approved by the institutional review boards of all five participating academic clinical centres. Participants provided informed consent prior to study entry. This paper reports results for the active treatment (randomised) phase of the study.
Participants
Participants were between 20 and 87 years old, referred for ECT, currently depressed and met Structured Clinical Interview for DSM–IV (SCID–I) Reference First, Spitzer, Gibbon and Williams20 criteria for primary major depressive disorder or bipolar disorder, with or without psychosis. Appropriateness for ECT was determined on a clinical basis after consultation with an attending-level ECT psychiatrist. Typical reasons for referral included multiple failed medication trials and severity/urgency of illness. Additional inclusion criteria were pre-treatment Hamilton Rating Scale for Depression–24 item (HRSD–24) Reference Hamilton21 total score ≥21, ability to cooperate in detailed neuropsychological testing, and to provide voluntary written informed consent.
Exclusion criteria were a lifetime diagnosis of schizophrenia, schizoaffective disorder or intellctual disabilities, recent (within the last year) diagnosis of anxiety disorder, obsessive–compulsive disorder, eating disorder that preceded the current episode of depression, current diagnosis of delirium, dementia, amnestic disorder or other central nervous system disease with the probability of affecting cognition or response to treatment, diagnosis (within 6 months) of active substance misuse/dependence, medical conditions contraindicating ECT, Mini-Mental State Examination (MMSE) Reference Folstein, Folstein and McHugh22 score ≤21 and ECT in the 6 months prior to the study.
Electrode placements
One of the following three electrode placements was used, depending upon the participant's group assignment: bifrontal, in which the centre of each electrode was placed 4–5 cm above the outer canthus of the eye along a vertical line perpendicular to a line connecting the pupils; Reference Letemendia, Delva, Rodenburg, Lawson, Inglis and Waldron8 bitemporal, in which the centre of the stimulus electrodes was applied 2–3 cm above the midpoint of the line connecting the outer canthus of the eye and the external auditory meatus on each side of the individual's head; and right unilateral, in which one electrode was positioned as in bitemporal on the right side (d'Elia placement). Reference d'Elia23 The centre of the other electrode was placed 2–3 cm to the right of the vertex of the skull. Standardisation of placement was assured by training of study psychiatrists at the initial investigators' meeting, use of an illustrative figure in each treatment suite and site visits by the study principal investigator.
ECT procedures
ECT procedures were standardised across all centres, using the Thymatron DGx ECT device (Somatics LLC, Lake Bluff, Illinois, USA), dose titration to determine seizure threshold at initial treatment and stimulus dosing at subsequent treatments as follows: one and a half times seizure threshold for bifrontal and bitemporal, six times seizure threshold for right unilateral (or at 100% of device maximum when six times seizure threshold could not be reached). Details of the stimulus algorithm used in the dose titration procedure to determine seizure threshold are shown in Table 1. Treatments were given three times a week, as is the clinical custom in the USA.
Table 1 Titration procedure
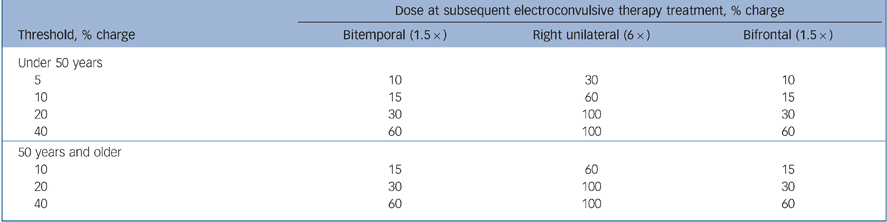
| Dose at subsequent electroconvulsive therapy treatment, % charge | |||
|---|---|---|---|
| Threshold, % charge | Bitemporal (1.5 ×) | Right unilateral (6 ×) | Bifrontal (1.5 ×) |
| Under 50 years | |||
| 5 | 10 | 30 | 10 |
| 10 | 15 | 60 | 15 |
| 20 | 30 | 100 | 30 |
| 40 | 60 | 100 | 60 |
| 50 years and older | |||
| 10 | 15 | 60 | 15 |
| 20 | 30 | 100 | 30 |
| 40 | 60 | 100 | 60 |
Procedures for anaesthesia and determination of seizure adequacy (electromyography (EMG) ≥20 sec; electroencephalogram (EEG)≥25) followed standardised clinical protocols compatible with current standards of care. 5 Anaesthesia management consisted of pre-treatment with glycopyrrolate, followed by induction with an anaesthetic agent (methohexital for 135 participants, thiopental for 75 participants, etomidate for 14 participants and propofol for 6), followed by succinylcholine for muscle relaxation. Participants were oxygenated throughout the procedure with 100% O2 with positive pressure delivered through a disposable bag and mask. Blood pressure, heart rate and pulse oximetry were monitored. Electroencephalogram was recorded from a single channel using left frontomastoid placements. Motor duration of seizures was recorded using a two-lead EMG from the right foot.
Masking procedure for electrode placements
In order to ensure that participants were unaware of which electrode placement was used, each person was prepared for all three types of electrode placement. This included placement of disposable electrode pads in bifrontal and bitemporal positions, and application of electrode gel to the vertex position. Only after the individual was unconscious was the designated electrode placement implemented.
Assessments
Instruments
The primary instrument used to rate depressive symptoms was the HRSD–24 administered at baseline and prior to each ECT treatment. The impact of electrode placement on neurocognitive performance was measured by an extensive battery of neuropsychological tests. The cognitive domains studied included orientation/global status, memory (verbal and non-verbal, anterograde and retrograde) and executive function. The specific instruments in the test battery were: the Mini-Mental State Examination (MMSE); the Rey Auditory Verbal Learning Test (AVLT); Reference Rey24,Reference Schmidt25 the Rey Osterrieth and the Taylor Complex Figure Tests; Reference Taylor26,Reference Rey and Osterrieth27 Autobiographical Memory Interview – Short Form (AMI–SF); Reference Sackeim, Prudic, Devanand, Kiersky, Fitzsimons and Moody28 the Trail Making Test, Reference Reitan29 Category Fluency, Reference Benton and Hamsher30 the Stroop Color Word Test, Reference Stroop31–Reference Strauss, Sherman and Spreen33 the Controlled Oral Word Association Test (COWAT), Reference Benton and Hamsher30 the Delis–Kaplan Executive Function System (D–KEFS) Sorting Test Reference Delis, Kaplan and Kramer34 and the Reading subtest of the Wide Range Achievement Test (WRAT–3). Reference Wilkinson35 Reorientation score 20 min after ECT was measured using a ten-question instrument, modified from an instrument previously used by the Columbia University group. Reference Sobin, Sackeim, Prudic, Devanand, Moody and McElhlney36 Global functioning was assessed using the Clinical Global Impression (CGI) scale. Reference Guy37
Raters
The raters who acquired study data were the study psychiatrist, the continuous rater and the neuropsychological technician. At specified time points (baseline and after the last ECT), the continuous rater and study psychiatrist each performed independent HRSD–24 ratings, with the mean of the ratings used for analyses. Raters were masked to treatment condition.
Outcome assessment
We used the longitudinal profile of continuous HRSD–24 total scores over the ECT treatment course (approximately three times a week) as one efficacy outcome. Other efficacy outcome measures were the single end-of-treatment HRSD–24 score and the proportion of remitters for each electrode placement group. The end-of-treatment HRSD–24 was obtained within 24–36 h after the final ECT, or as soon thereafter as possible. Remitter criteria were: a ≥60% decrease from baseline in HRSD–24 total score; HRSD–24 ≤10 on two consecutive ratings; and HRSD–24 did not change >3 points on the last two consecutive treatments. A specific minimum or maximum number of ECT was not required for an individual to be classified as a remitter. People who did not meet remission criteria and who received at least ten treatments were declared non-remitters. Participants were considered to have dropped out of the study if consent for ECT or study participation was withdrawn before ten ECT had been administered or initial seizure threshold was 80% or higher, or ECT was discontinued for clinical reasons before ten ECT had been administered. Response was defined as a decrease in HRSD–24 total score of 50% from baseline.
Statistical analyses
All statistical tests were carried out using SAS version 9.13 for Windows.
Descriptive analyses
Continuous demographic and baseline clinical characteristics were compared across electrode placement (treatment) groups using a generalised linear models approach or Kruskal–Walis one-way ANOVA; categorical variables were compared using chi-squared analyses.
Missing data
Missing data occurred for the continuous HRSD–24 outcome if the participant did not return for the final HRSD–24 assessment within 24–36 h after the final ECT. Because there was no prescribed number of ECT for remitters, the final ECT was the last treatment received regardless of time in the study. Analyses involving the full longitudinal profile of HRSD–24 values did not require imputation of missing values because the analysis method (mixed effects modelling) can accommodate missing data. For analyses of the single end-of-treatment measure, the HRSD–24 obtained immediately prior to (e.g. on the morning of) the final ECT was used as the missing end-of-treatment value. This occurred for 40 participants (17%). Missing outcomes for neurocognitive test battery results were imputed using multiple imputation (SAS Proc MI).
Efficacy analyses
The efficacy analyses used a modified intent-to-treat (ITT) sample comprising all randomised participants who had at least one post-baseline assessment. In analyses of the continuous efficacy outcome, the longitudinal trajectories of HRSD–24 scores over the treatment course were compared among electrode placement groups using a mixed effects modelling approach (SAS Proc Mixed). Reference Hedeker and Gibbons38 The auto-regressive covariance structure was used because it resulted in the best fit for the mixed effects modelling. A series of models was evaluated beginning with the simple model containing only treatment, time and treatment × time interaction effects as independent variables (simple or unadjusted model). Addition of psychosis status, polarity, age and clinical centre to the mixed effects modelling provided a comparison of electrode placements adjusted for these covariates (adjusted model). Psychosis status, polarity and clinical centre were included as covariates because they were stratification variables in the randomisation. In addition, we explored inclusion of a random intercept and a quadratic time trend in the model. Both the linear and quadratic terms in the polynomial model were statistically significant and the quadratic model (simple and adjusted) was used as the final analysis model. Pair-wise comparisons of mixed effects modelling least squares means were adjusted using the Tukey–Kramer multiple comparison procedure.
In another set of analyses, the mean end-of-treatment HRSD–24 total scores (single end-point) were compared among the treatment (electrode placement) groups using a general linear models approach. The adjusted general linear model contained the same covariates as described for mixed effects modelling and post hoc pair-wise comparisons of least squares means between electrode placement groups were carried out using the Tukey–Kramer multiple comparison procedure.
Paired t-tests were used to evaluate change from baseline within each electrode placement group.
Remission proportions were estimated for each electrode placement using 95% confidence intervals. For the repeated measures (longitudinal) analyses, we have 90% power to detect a standardised effect size of approximately 0.24–0.32 standard deviations in pair-wise comparisons between electrode placement groups (assuming two-sided level of significance α = 0.05, number of repeated measures: six based on average number of ECT administered, and intraclass correlation (ICC) ranging from 0.3 to 0.7). Based on our estimated common pooled standard deviation for HRSD–24 total scores of 8.87, this is equivalent to a raw effect size that can be detected of 2.1–2.8 units on the HRSD–24 scale. For the continuous single end-point HRSD–24 outcome, the study had 85% power to detect effect sizes of approximately 4.5 HRSD–24 units (0.5 standardised units) or higher.
Cognitive analyses
The continuous end-of-active treatment neuropsychological variables were analysed using the multivariable general linear models approach (SAS Proc GLM (general linear model)). The adjusted general linear model contained the baseline level of the given instrument, age, gender, psychosis, polarity, clinical centre, last HRSD–24 and WRAT–3 as covariates. The last HRSD–24 score was used to adjust for level of illness severity at the time the neuropsychological variables were assessed. The WRAT–3 was included as a measure of pre-treatment intellectual functioning. Post hoc pair-wise comparisons of least squares means between electrode placement groups were carried out using Tukey's multiple comparison procedure. The cognitive analyses have 85% power to detect standardised effect sizes in pair-wise treatment comparisons ranging from 0.5 to 0.6 for imputed sample sizes ranging from approximately 70 (MMSE) to 56 (D–KEFS) per group. Sample sizes per instrument differed based on number of participants missing baseline levels for a given instrument, and therefore eliminated from the modified ITT sample size for the cognitive analyses of that neuropsychological variable. The amount of missing data that required imputation for the neuropsychological instruments ranged from 35 to 55% by the end of acute treatment. The percentage missing baseline data for the instrument (and hence eliminated from the imputed data-set) ranged from 9 to 25%. Missingness for these variables was largely attributable to participant refusal or lack of time to administer the battery. Although multiple imputation methods were used to impute the missing values, caution is exercised in the interpretation of the cognitive results because of the amount of data that had to be estimated, as well as the missing baseline values.
Results
Participant flow and characteristics
Figure 1 describes the flow of participants through the study. A total of 274 people were entered into the study; 37 screen failures were excluded after entry so 237 people were randomised. Of these, 7 had no post-baseline assessment, yielding a modified ITT efficacy evaluable sample of 230 individuals: 77 right unilateral, 81 bifrontal and 72 bitemporal. Among the modified ITT sample, 63 of 230 participants (27.4%) exited the study early. There were no statistically significant differences in demographic or baseline clinical characteristics between completers and those who dropped out.

Fig. 1 Participant flow.
ECT, electroconvulsive therapy.
a. Modified ITT sample (a priori defined).
b. Participant perception or clinician determined.
The rate of drop out was similar across all three groups; 31.2% for right unilateral, 27.2% for bifrontal and 23.6% for bitemporal (P = 0.581). Major reasons for dropping out across the three treatment groups were confusion/cognitive impairment (19.3%), ECT not working (17.5%), non-cognitive side-effect (7.0%) and improvement in condition (5.3%). There were no statistically significant differences between the groups for any of these reasons for drop out.
For the ITT sample, 63.5% were female, 95.5% were White and the mean age was 53.1 (s.d. = 15.0) years. Of the sample, 23.5% had psychotic features and 22.7% had bipolar depression. The mean baseline HRSD–24 score was 34.6 (s.d. = 7.2). There was no difference in psychosis status, polarity and baseline HRSD–24 between the groups (Table 2).
Table 2 Participant characteristics for the intent-to-treat sample and by treatment
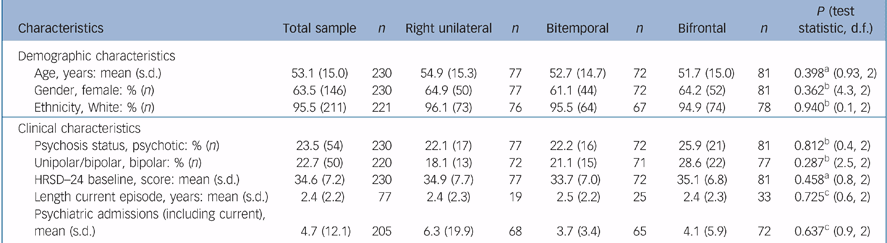
| Characteristics | Total sample | n | Right unilateral | n | Bitemporal | n | Bifrontal | n | P (test statistic, d.f.) |
|---|---|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||||
| Age, years: mean (s.d.) | 53.1 (15.0) | 230 | 54.9 (15.3) | 77 | 52.7 (14.7) | 72 | 51.7 (15.0) | 81 | 0.398a (0.93, 2) |
| Gender, female: % (n) | 63.5 (146) | 230 | 64.9 (50) | 77 | 61.1 (44) | 72 | 64.2 (52) | 81 | 0.362b (4.3, 2) |
| Ethnicity, White: % (n) | 95.5 (211) | 221 | 96.1 (73) | 76 | 95.5 (64) | 67 | 94.9 (74) | 78 | 0.940b (0.1, 2) |
| Clinical characteristics | |||||||||
| Psychosis status, psychotic: % (n) | 23.5 (54) | 230 | 22.1 (17) | 77 | 22.2 (16) | 72 | 25.9 (21) | 81 | 0.812b (0.4, 2) |
| Unipolar/bipolar, bipolar: % (n) | 22.7 (50) | 220 | 18.1 (13) | 72 | 21.1 (15) | 71 | 28.6 (22) | 77 | 0.287b (2.5, 2) |
| HRSD—24 baseline, score: mean (s.d.) | 34.6 (7.2) | 230 | 34.9 (7.7) | 77 | 33.7 (7.0) | 72 | 35.1 (6.8) | 81 | 0.458a (0.8, 2) |
| Length current episode, years: mean (s.d.) | 2.4 (2.2) | 77 | 2.4 (2.3) | 19 | 2.5 (2.2) | 25 | 2.4 (2.3) | 33 | 0.725c (0.6, 2) |
| Psychiatric admissions (including current), mean (s.d.) | 4.7 (12.1) | 205 | 6.3 (19.9) | 68 | 3.7 (3.4) | 65 | 4.1 (5.9) | 72 | 0.637c (0.9, 2) |
Efficacy results
The change in HRSD–24 outcomes from baseline to the end of treatment within each electrode placement group demonstrated that all three placements were highly effective treatments. The mean change from baseline for HRSD–24 total scores (baseline to end) was greater than 20 points in all three groups (P<0.0001, all groups by paired t-test; Table 3).
Table 3 HRSD–24 outcomes by electrode placementa
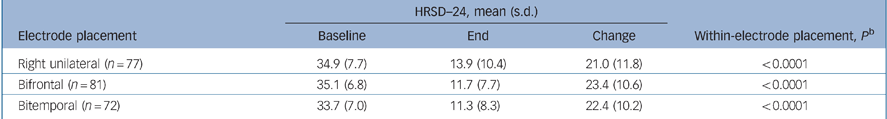
| HRSD—24, mean (s.d.) | ||||
|---|---|---|---|---|
| Electrode placement | Baseline | End | Change | Within-electrode placement, P b |
| Right unilateral (n = 77) | 34.9 (7.7) | 13.9 (10.4) | 21.0 (11.8) | < 0.0001 |
| Bifrontal (n = 81) | 35.1 (6.8) | 11.7 (7.7) | 23.4 (10.6) | < 0.0001 |
| Bitemporal (n = 72) | 33.7 (7.0) | 11.3 (8.3) | 22.4 (10.2) | < 0.0001 |
The trajectory of observed means over the ECT treatment course is demonstrated in Fig. 2. The trajectory is steep for all placements up to visit six (after five ECT). The flattening of the trajectories after approximately six ECT (visit seven) reflects the relatively early remissions for all placement groups (among 137 remitters, 74% of bitemporal, 69% of right unilateral and 59% of bifrontal achieved remission with six or fewer ECT).
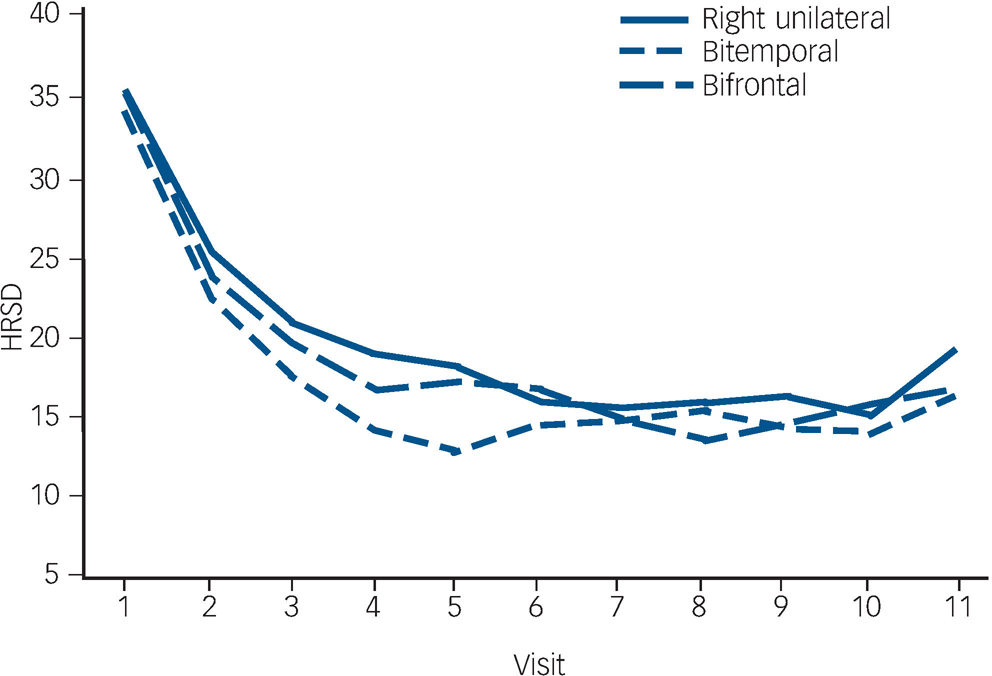
Fig. 2 Observed Hamilton Rating Scale for Depression–24 (HRSD–24) total score means.
In mixed effects modelling longitudinal analyses of trajectories of HRSD–24 total scores over the ECT treatment course, there was a significant downwards trend for all electrode placements in the unadjusted and adjusted polynomial models (coefficients for linear and quadratic time effects for both models: Ps<0.0001) (Fig. 3). The model-fitted HRSD–24 means for bitemporal placement were significantly lower than those for right unilateral at visits two to eight (after ECT one to seven). The difference in covariate-adjusted HRSD–24 means between the bitemporal and right unilateral placements was approximately three HRSD–24 units over these time periods (visit two: difference in least squares means effect size (ES) = 2.54, Tukey–Kramer adjusted P = 0.058); visit three: ES = 2.90, adjusted P = 0.016; visit four: ES = 3.14, adjusted P = 0.013; visit five: ES = 3.25, adjusted P = 0.016; visit six: ES = 3.26, adjusted P = 0.022; visit seven: ES = 3.13, adjusted P = 0.037; visit eight: ES = 2.88, adjusted P = 0.085). The HRSD–24 means for bitemporal placement were significantly lower than those for bifrontal at visits three to five (after ECT two to four) (visit three: ES = 2.45, adjusted P = 0.047; visit four: ES = 2.60, adjusted P = 0.046; visit five: ES = 2.51, adjusted P = 0.081). The HRSD–24 means did not differ significantly for right unilateral compared with bifrontal placement at any time point. Among those who remitted, almost all (≥90%) of the remissions occurred within approximately 3 weeks of treatment (≤9 ECT). Participants remaining in the study up to visit nine were predominantly those for whom none of the treatments were effective (non-remitters). In further mixed effects modelling analyses, we restricted interest to the time period in which the early rapid decrease in symptoms occurred (e.g. after approximately 2 weeks of treatment). For this period, the rate of decrease in HRSD–24 scores for the bitemporal placement was significantly greater than that for right unilateral, indicating a more rapid rate of symptom reduction for this placement (bitemporal v. right unilateral: P = 0.029/0.026 for linear/quadratic terms in adjusted mixed effects modelling). Further, the bifrontal placement produced a decrease in symptom severity that was marginally significantly better than that of right unilateral over the early treatment period (bifrontal v. right unilateral: P = 0.109/0.084 for linear/quadratic terms in adjusted mixed effects modelling).
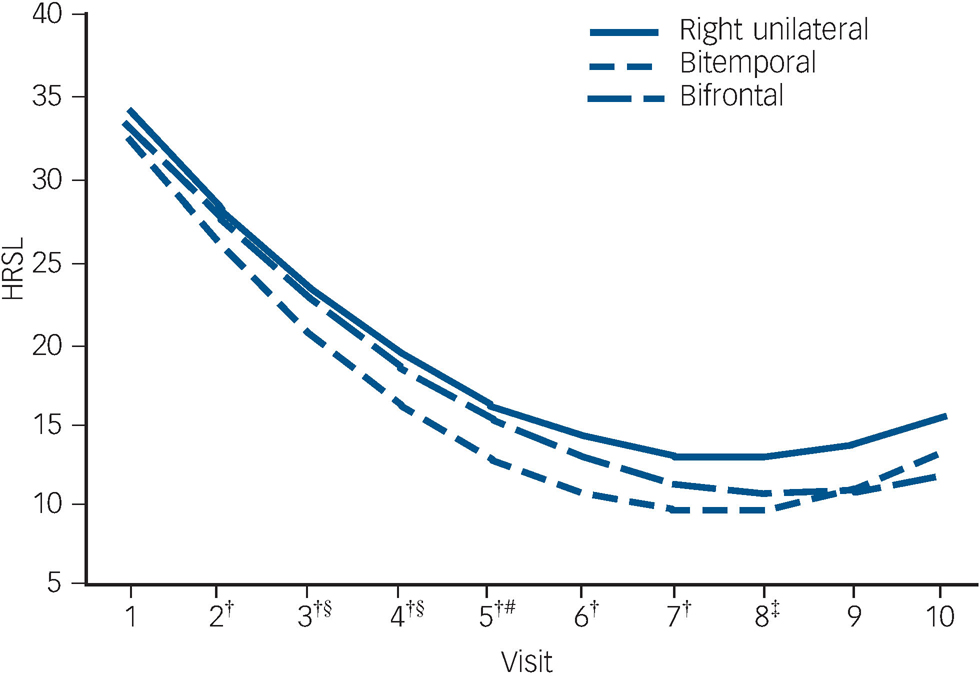
Fig. 3 Fitted Hamilton Rating Scale for Depression–24 (HRSD–24) total score means from longitudinal mixed models analysis with linear and quadratic terms for time.
†Bitemporal v. right unilateral: 0.01≤P≤0.058; ‡bitemporal v. right unilateral: P = 0.085; §bitemporal v. bifrontal: P<0.05; #bitemporal v. bifrontal: P = 0.081.
After only one ECT, there was a 10.6 (s.d. = 8.6) point reduction, on average, in symptom severity (decline in HRSD–24 total scores) for the three electrode placements combined. This early reduction in symptom severity after only one ECT represented approximately 48% (10.63/22.29) of the total decline in HRSD–24 scores over the full treatment period. The reduction in severity after one ECT within each electrode placement was: right unilateral 44.1% (9.28/21.03); bifrontal 47.7% (11.17/23.40); bitemporal 51.1% (11.45/22.40). The decline in HRSD–24 total scores after the first ECT was marginally greater for bitemporal compared with right unilateral (bitemporal v. right unilateral, P = 0.073 from general linear models adjusted for baseline HRSD–24, age, clinical centre, psychosis status, polarity). Comparisons of these early declines for right unilateral v. bifrontal and for bitemporal v. bifrontal were not statistically significant (right unilateral v. bifrontal, P = 0.251; bifrontal v. bitemporal, P = 0.791, from covariate-adjusted general linear models)
Considering the single end-of-treatment value for all participants (remitters, non-remitters, individuals who dropped out), there were no statistically significant differences between HRSD–24 end scores among electrode placement groups after adjustment for baseline HRSD–24, site, age, psychosis and polarity (right unilateral: 13.1 (95% CI 11.1–15.2); bifrontal: 11.5 (95% CI 9.5–13.5); and bitemporal 11.4 (95% CI 9.3–13.5), P = 0.418, general linear models analyses). It should be noted that the study was adequately powered to detect effect sizes for the continuous single end-point HRSD–24 outcome of approximately 4.5 HRSD–24 units or higher. The effect sizes that can be detected with the longitudinal analyses are smaller than those for single end-point analyses for a given level of power.
Table 4 and Fig. 4 present remission outcomes at the end of the acute course of ECT for each electrode placement. Based on 95% confidence interval estimation, population remission proportions for right unilateral were estimated to range from 43 to 66%; for bifrontal estimates range from 50 to 71%; and for bitemporal, the estimates range from 53 to 75%. These remission proportion confidence interval estimates apply to the potential population of all individuals who may receive the treatments, taking into account the uncertainty in the sampling process. Post hoc power analyses for the remission outcomes indicate low power for detecting significant differences between electrode placement groups, therefore attention should be focused on estimation of the proportions via 95% confidence intervals rather than hypothesis testing (P-values).
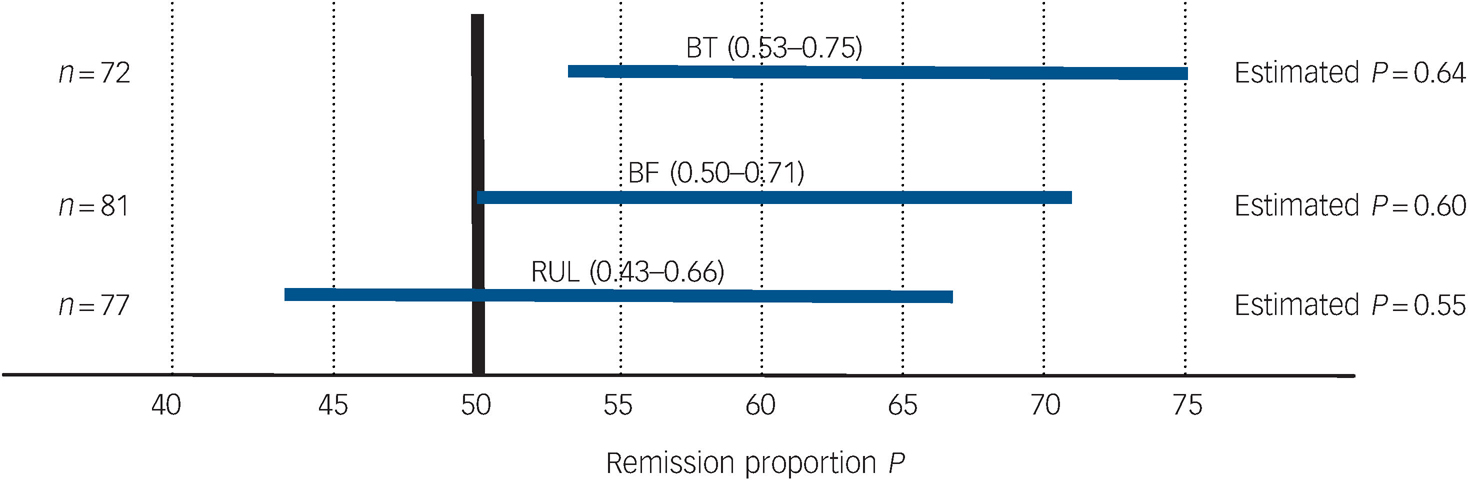
Fig. 4 95% CI estimates of remission proportions for bitemporal (BT), bifrontal (BF) and right unilateral (RUL) electrode placements.
Table 4 Remission outcome by electrode placementa

| Electrode placement | Remitted, % (n) | Non-remitted, % (n) | Dropped out,b % (n) | Total, n |
|---|---|---|---|---|
| Right unilateral | 54.6 (42) | 14.3 (11) | 31.2 (24) | 77 |
| Bifrontal | 60.5 (49) | 12.4 (10) | 27.2 (22) | 81 |
| Bitemporal | 63.9 (46) | 12.5 (9) | 23.6 (17) | 72 |
Global functioning, as assessed by the CGI severity scale, mirrored the HRSD results, with the bitemporal group less ill (mean = 2.32, s.d. = 1.43) than the bifrontal (mean = 2.48, s.d. = 1.30) or right unilateral (mean = 2.86, s.d. = 1.60) (unadjusted means comparison P = 0.07, model adjusted P = 0.19) at the end of the treatment course.
The mean number of ECT among remitters was 5.9 (s.d. = 2.3) for right unilateral, 6.2 (s.d. = 2.6) for bifrontal and 5.5 (s.d. = 2.3) for bitemporal placement (P = 0.405 from general linear models).
Cognitive results
There were no significant differences between the electrode placement groups for the instruments measuring overall global cognitive function (MMSE) and executive function (Category Fluency, COWAT, Stroop, Trail Making A, B and D–KEFS) (Tables 5 and 6). Bifrontal placement was statistically significantly inferior to bitemporal on two measures of anterograde memory (AVLT 1–5, AVLT Delay) and showed a trend towards inferiority (P = 0.10) on a measure of anterograde memory (AVLT%) and retrograde amnesia (AMI). Right unilateral placement was not statistically significantly superior to the bilateral placements on any of these cognitive measures.
Table 5 Memory function tests and Mini-Mental State Examinationa
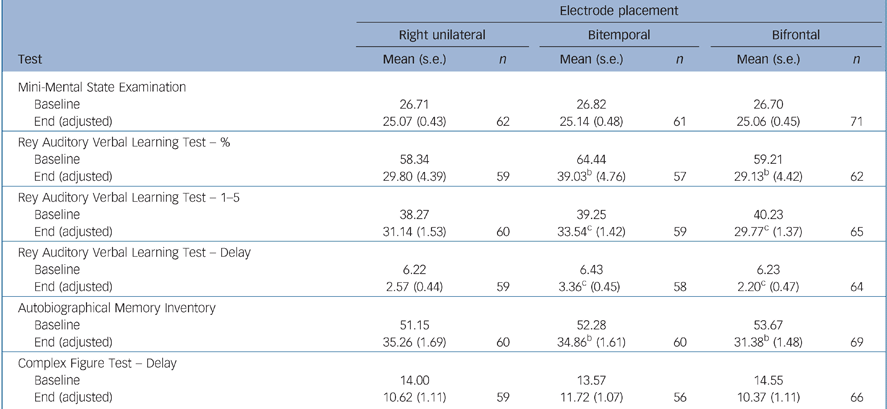
| Electrode placement | ||||||
|---|---|---|---|---|---|---|
| Right unilateral | Bitemporal | Bifrontal | ||||
| Test | Mean (s.e.) | n | Mean (s.e.) | n | Mean (s.e.) | n |
| Mini-Mental State Examination | ||||||
| Baseline | 26.71 | 26.82 | 26.70 | |||
| End (adjusted) | 25.07 (0.43) | 62 | 25.14 (0.48) | 61 | 25.06 (0.45) | 71 |
| Rey Auditory Verbal Learning Test — % | ||||||
| Baseline | 58.34 | 64.44 | 59.21 | |||
| End (adjusted) | 29.80 (4.39) | 59 | 39.03b (4.76) | 57 | 29.13b (4.42) | 62 |
| Rey Auditory Verbal Learning Test — 1-5 | ||||||
| Baseline | 38.27 | 39.25 | 40.23 | |||
| End (adjusted) | 31.14 (1.53) | 60 | 33.54c (1.42) | 59 | 29.77c (1.37) | 65 |
| Rey Auditory Verbal Learning Test — Delay | ||||||
| Baseline | 6.22 | 6.43 | 6.23 | |||
| End (adjusted) | 2.57 (0.44) | 59 | 3.36c (0.45) | 58 | 2.20c (0.47) | 64 |
| Autobiographical Memory Inventory | ||||||
| Baseline | 51.15 | 52.28 | 53.67 | |||
| End (adjusted) | 35.26 (1.69) | 60 | 34.86b (1.61) | 60 | 31.38b (1.48) | 69 |
| Complex Figure Test — Delay | ||||||
| Baseline | 14.00 | 13.57 | 14.55 | |||
| End (adjusted) | 10.62 (1.11) | 59 | 11.72 (1.07) | 56 | 10.37 (1.11) | 66 |
Table 6 Executive function testsa
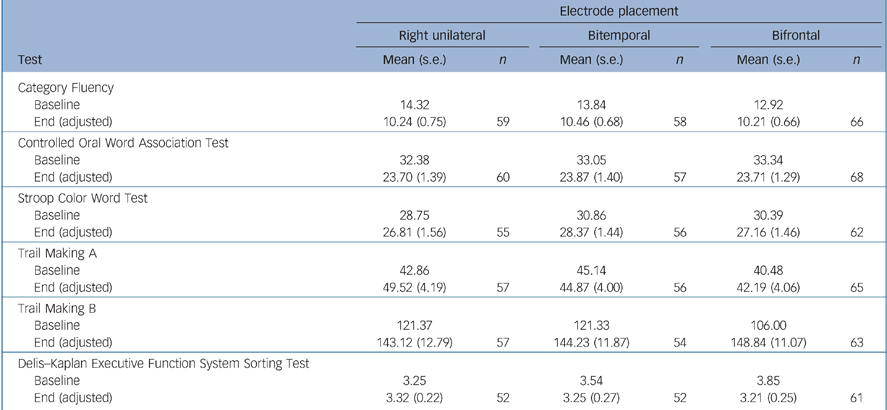
| Electrode placement | ||||||
|---|---|---|---|---|---|---|
| Right unilateral | Bitemporal | Bifrontal | ||||
| Test | Mean (s.e.) | n | Mean (s.e.) | n | Mean (s.e.) | n |
| Category Fluency | ||||||
| Baseline | 14.32 | 13.84 | 12.92 | |||
| End (adjusted) | 10.24 (0.75) | 59 | 10.46 (0.68) | 58 | 10.21 (0.66) | 66 |
| Controlled Oral Word Association Test | ||||||
| Baseline | 32.38 | 33.05 | 33.34 | |||
| End (adjusted) | 23.70 (1.39) | 60 | 23.87 (1.40) | 57 | 23.71 (1.29) | 68 |
| Stroop Color Word Test | ||||||
| Baseline | 28.75 | 30.86 | 30.39 | |||
| End (adjusted) | 26.81 (1.56) | 55 | 28.37 (1.44) | 56 | 27.16 (1.46) | 62 |
| Trail Making A | ||||||
| Baseline | 42.86 | 45.14 | 40.48 | |||
| End (adjusted) | 49.52 (4.19) | 57 | 44.87 (4.00) | 56 | 42.19 (4.06) | 65 |
| Trail Making B | ||||||
| Baseline | 121.37 | 121.33 | 106.00 | |||
| End (adjusted) | 143.12 (12.79) | 57 | 144.23 (11.87) | 54 | 148.84 (11.07) | 63 |
| Delis—Kaplan Executive Function System Sorting Test | ||||||
| Baseline | 3.25 | 3.54 | 3.85 | |||
| End (adjusted) | 3.32 (0.22) | 52 | 3.25 (0.27) | 52 | 3.21 (0.25) | 61 |
Reorientation score at 20 min measured at ECT session one was statistically better for right unilateral v. the other two electrode placements (right unilateral: 8.0 (s.d. = 3.1); bifrontal: 4.6 (s.d. = 3.6); and bitemporal: 6.0 (s.d. = 3.9); right unilateral v. bifrontal P<0.0001, right unilateral v. bitemporal P = 0.007; and bitemporal v. bifrontal P = 0.091; from general linear model adjusted for site and age with Tukey correction for multiple comparisons). Since ECT session one is the dose titration session, right unilateral is not administered at close to the stimulus dose (six times seizure threshold) used at subsequent treatments. Reorientation score at 20 min after ECT session two showed that right unilateral maintained its advantage over bifrontal (right unilateral: 5.9 (s.d. = 3.3); bifrontal: 4.3 (s.d. = 3.0) (P = 0.010)), but was not statistically different from bitemporal placement (bitemporal: 5.8 (s.d. = 3.4) (P = 0.952)). Bitemporal was statistically superior to bifrontal in reorientation score after treatment session two (P = 0.026). Averaged across all ECT sessions, but excluding ECT session one, the three electrode placements were not statistically different, but their relative order remained the same as for ECT session two (right unilateral: 5.7 (s.d. = 2.5); bitemporal: 5.5 (s.d. = 2.8); bifrontal: 4.8 (s.d. = 2.5); right unilateral v. bifrontal P = 0.113; right unilateral v. bitemporal P = 0.901; bitemporal v. bifrontal P = 0.267, from general linear models adjusted for site and age with Tukey correction for multiple comparisons).
Discussion
Efficacy
Each electrode placement resulted in clinically and statistical significance decreases in depression severity. Bitemporal electrode placement resulted in a more rapid decrease in symptom severity, early in the course of treatment. Each of the three placements resulted in a substantial decrease in symptoms with the initial treatment.
These results are consistent with several decades of data comparing antidepressant outcomes between bitemporal and right unilateral placement, and add important data about the more recently developed bifrontal placement. Two other randomised controlled trials that compared right unilateral (administered in a similar way to the present study) and bitemporal remission rates 1 week after the ECT course also found inferior rates for right unilateral placement (60% v. 65%, Reference Sackeim, Prudic, Devanand, Kiersky, Fitzsimons and Moody10 59% v. 65%) Reference Sackeim, Prudic, Devanand, Nobler, Lisanby and Peyser9,Reference Sackeim, Prudic, Nobler, Fitzsimons, Lisanby and Payne39 that did not reach statistical significance. Our right unilateral efficacy data should be interpreted in the context of its administration at the six times seizure threshold, a relatively recent technical enhancement that is believed to optimise this electrode placement. It should also be noted that US ECT devices are limited to a charge output of under 600 mC, Reference Lisanby, Devanand, Nobler, Prudic, Mullen and Sackeim40 preventing a small number of the participants (5/77, 6.5%) in this study from being treated at fully six times seizure threshold. Both bilateral placements resulted in slightly, but not significantly, superior remission proportions than right unilateral placements. It is possible that with increased power to detect differences with an even larger sample size, a potentially meaningful clinical difference favouring the bilateral placements would also become statistically significant. Based on our results, particularly the superior speed of response seen with bitemporal electrode placement, it is appropriate to continue the preferential use of bitemporal electrode placement in more urgent clinical situations. Such situations might include high suicide risk, severe medical comorbidities and catatonia. On the other hand, right unilateral at high stimulus doses should be considered an effective form of ECT. When the practitioner and individual are most concerned about minimising retrograde amnesia, right unilateral may be the preferred initial choice, given the accumulated evidence in the literature of its more benign cognitive profile. The suggestion by Prudic Reference Prudic17 that right unilateral electrode placement may be more rapidly effective than bitemporal was not supported by our findings.
Our data demonstrating the substantial impact on depressive symptoms of the initial treatment in the series are also consistent with prior reports in the literature. Reference Keisling41–Reference Thomas and Kellner43 However, the fact that right unilateral placement was administered at a near threshold dose (the first treatment was the one at which the dose titration procedure to estimate seizure threshold was carried out) is intriguing, given observations that right unilateral may need to be given at multiples of seizure threshold to insure efficacy. Reference Sackeim, Prudic, Devanand, Kiersky, Fitzsimons and Moody10,Reference McCall, Reboussin, Weiner and Sackeim12
Cognition
Our cognitive function data reveal few differences between the electrode placements on a variety of neuropsychological instruments. Bifrontal electrode placement was developed upon the theoretical assumption that moving the stimulus electrodes farther from the temporal lobes (particularly the hippocampi) would result in less memory impairment. On the other hand, seizure initiation from the frontal lobes beneath the bifrontally placed electrodes might be theorised to produce more executive dysfunction. Our data neither confirm a memory advantage for bifrontal (in fact, on some measures they show a disadvantage), nor a disadvantage for executive functioning. Bifrontal placement has become quite commonly used based on prior reports of its efficacy and side-effect profiles Reference Letemendia, Delva, Rodenburg, Lawson, Inglis and Waldron8,Reference Bailine, Rifkin, Kayne, Selzer, Vital-Herne and Blieka13 and also because of its ease of use in practice. However, the evidence base in the literature for bifrontal remains much smaller than that for either bitemporal or right unilateral, and some would continue to regard it an as experimental placement.
Right unilateral electrode placement was developed based upon the theoretical assumption that sparing the language centres of the left hemisphere the direct passage of the electrical stimulus would result in less cognitive impairment. Surprisingly, in our study, right unilateral was not consistently superior to bitemporal except for reorientation 20 min after ECT. Sobin et al suggest that speed of reorientation after ECT is a proxy for longer term memory impairment. Reference Sobin, Sackeim, Prudic, Devanand, Moody and McElhlney36 Our failure to find a consistently superior cognitive profile for right unilateral placement may be a result of administering right unilateral at high stimulus doses, a technique that may diminish the cognitive advantages of this placement when administered at lower stimulus doses. Reference McCall, Dunn, Rosenquist and Hughes44 We cannot eliminate the possibility that failure to find a cognitive advantage of one placement over another may be the result of undetected bias caused by differential rates of drop out in those participants with the worst cognitive outcomes. Further study to better characterise the specific cognitive profile of each electrode placement is clearly warranted, including more frequent measurement time points, and longer study periods to characterise the time course of resolution of cognitive effects. We advocate the development of a streamlined, ECT-specific neuropsychological assessment battery that requires a considerably shortened administration time, and can be administered concurrent with the illness symptom severity instruments. This would allow the concurrent tracking of illness severity and cognitive changes, and potentially would allow disentangling of these effects. Further, a considerable reduction in administration time should dramatically reduce the amount of missing data resulting in more reliable cognitive results.
Limitations
Failure of the study to find statistically significant differences for both efficacy and cognitive outcomes cannot be taken to mean that the outcomes in the two groups are equal. Lack of such differences could be the result of low statistical power, particularly for the cognitive outcome variables for which sample sizes were substantially reduced.
Implications
Our data add to the evidence base that right unilateral at six times seizure threshold and bifrontal and bitemporal at one and a half times seizure threshold are all highly efficacious electrode placements for use in ECT for the treatment of major depression. Practitioners may be reassured that each standard electrode placement in contemporary ECT practice, when given with appropriate electrical stimulus dosing, is a highly effective antidepressant technique. Because bitemporal placement results in more rapid depressive symptom reduction, it is the preferred electrode placement when the clinical situation requires urgent improvement. Our data do not support a cognitive advantage of bifrontal over bitemporal placement.
Acknowledgements
We thank the entire CORE study team, especially: Hilary Bernstein, Melanie Biggs, Margaret Sinex, Chitra Malur, M. Kevin O'Connor, Rafik Istafanous, Lisa Jakubowski, Zainab Ali, Monica Mendez, Christopher Phillips, Judy Shaw, Donna Devine, Kelly Jacobson and Teresa Rummans.













eLetters
No eLetters have been published for this article.