Long-term prescription opioid analgesic use (OAU) for chronic non-cancer pain is defined as ‘daily or near-daily’ use for >90 days.Reference Chou1, Reference Von Korff, Saunders, Thomas Ray, Boudreau, Campbell and Merrill2 Between 1.4 and 10% of patients with a new opioid prescription develop chronic OAU,Reference Von Korff, Saunders, Thomas Ray, Boudreau, Campbell and Merrill2, Reference Scherrer, Salas, Copeland, Stock, Ahmedani and Sullivan3 and a majority, 65–80%, of patients who have >90 days OAU, are still taking opioids 3–5 years later.Reference Vanderlip, Sullivan, Edlund, Martin, Fortney and Austen4, Reference Martin, Fan, Edlund, Devries, Brennan-Braden and Sullivan5 These patients are more likely than those who take opioids short term to develop opioid use disorder and overdose. Chronic OAU is also associated with new depressive episodes (NDEs)Reference Scherrer, Salas, Copeland, Stock, Ahmedani and Sullivan3, Reference Scherrer, Salas, Sullivan, Schneider, Bucholz and Burroughs6, Reference Scherrer, Svrakic, Freedland, Chrusciel, Balasubramanian and Bucholz7 and treatment-resistant depression.Reference Scherrer, Salas, Sullivan, Schneider, Bucholz and Burroughs6 Because depression and OAU are mutually reinforcing,Reference Sullivan8 these patients may be in a cycle of persistent OAU, depression and pain. Research on treating depression to improve outcomes for chronic non-cancer pain is sparse. In Kroenke et al'sReference Kroenke, Bair, Damush, Wu, Hoke and Sutherland9 Stepped Care for Affective Disorders and Musculoskeletal Pain (SCAMP) study, where patients were randomised to optimal depression treatment v. usual care, treating depression led to reduced pain severity and anxiety, improved functioning and better health-related quality of life.Reference Kroenke, Bair, Damush, Wu, Hoke and Sutherland9 Although SCAMP was not designed to measure change in OAU, the findings raise the possibility that depression treatment could reduce use of opioids, possibly from reduced pain or improved functioning.Reference Kroenke, Bair, Damush, Wu, Hoke and Sutherland9–Reference Vowles, Witkiewitz, Levell, Sowden and Ashworth11 Improved functioning can occur independent of painReference Vowles, Witkiewitz, Levell, Sowden and Ashworth11 and should follow depression treatment. Independent of changes in pain severity, the need for OAU to self-regulate mood should dissipate following reduction in depression.
We are not aware of any studies that report changes in OAU following adherence to antidepressant medication (ADM) treatment in patients with chronic non-cancer pain. Using a retrospective cohort design, it is possible to test the hypothesis that adherence to ADM treatment v. non-adherence is associated with OAU cessation without the ethical barrier of randomising to inadequate treatment. Adherence serves as an indicator of depression improvement because patients who are non-adherent are less likely to have decreasing depression symptoms.Reference Ho, Chong, Chaiyakunapruk, Tangiisuran and Jacob12 Among patients initiating ADMs, response to treatment by 24 weeks is much lower in non-adherent v. adherent patients (55.8 v. 82.5%).Reference Akerblad, Bengtsson, von Knorring and Ekselius13 Although it would be ideal to have 9-item Patient Health Questionnaire (PHQ-9)Reference Kroenke, Spitzer and Williams14 scores for all patients at the time of ADM initiation and opioid cessation, such data was available from only a subset of patients. Therefore, we used adherence as a proxy for depression improvement. In a large cohort of Veterans Health Administration patients with NDE following >90 days of OAU, we tested the hypothesis that depression treatment adherence was associated with OAU cessation. Specifically, the objective of the current study was to determine whether patients who developed depression following chronic OAU were more likely to stop using opioids if they adhered to ADM treatment compared with patients who did not adhere to ADM treatment. In addition, exploratory analysis in a subset of patients with sufficient data was computed to assess the change in depression symptoms and pain scores over time in patients adherent to treatment with ADM compared with those who were non-adherent who did and did not stop OAU.
Method
This retrospective cohort analysis used patient data extracted from the Veterans Health Administration electronic medical record for 1 Jan 2000 to 31 Dec 2012. Data included ICD-9-CM diagnostic codes,15 in-patient stays, out-patient visits, prescriptions dispensed records, vital signs and demographic information.
Cohort identification
A random sample of 500 000 patients was taken from a cohort of 2 910 335 identified with at least one out-patient visit in both fiscal years 1999 and 2000, and aged 18–80 years. We excluded patients over 80 because they are more likely to receive prescription opioids for end-of-life pain management and cancer pain and the risk of misclassifying depression increases because of the greater prevalence of vascular depression and depression related to dementia. From this sample, we excluded 151 500 patients with a cancer and/or HIV diagnosis. Patients must have had at least one yearly visit in the 2-year ‘washout’ period (2000–2001) during which they must have been free of a medical record depression diagnosis (n = 266 901). We then selected patients with a NDE beginning in 2002–2011 and not occurring on the last out-patient visit date (n = 31 224). Because our previous reports indicate >90-day OAU is associated with up to twice the risk of NDEs,Reference Scherrer, Salas, Copeland, Stock, Ahmedani and Sullivan3, Reference Scherrer, Svrakic, Freedland, Chrusciel, Balasubramanian and Bucholz7 we limited the cohort to patients with >90 days of OAU or by the date of the NDE (n = 3075).
NDE was defined by the presence of a primary diagnosis (ICD-9-CM: 296.2, 296.3, 311) of depression in at least one in-patient stay or two out-patient visits within the same 12-month period. This algorithm has been shown to be a valid measure of depression when compared with self-report or written medical record information.Reference Frayne, Miller, Sharkansky, Jackson, Wang and Halanych16, Reference Solberg, Engebretson, Sperl-Hillen, Hroscikoski and O'Connor17 Patients without ADM treatment on or after the NDE were excluded (n = 138). Patients must have had >3 months follow-up after NDE diagnosis to allow for the possibility of the occurrence of least one acute-phase depression treatment period (≥84 days)18 (n = 2843). The final sample included patients with complete demographic data (n = 2821). The cohort selection process is shown in Fig. 1.
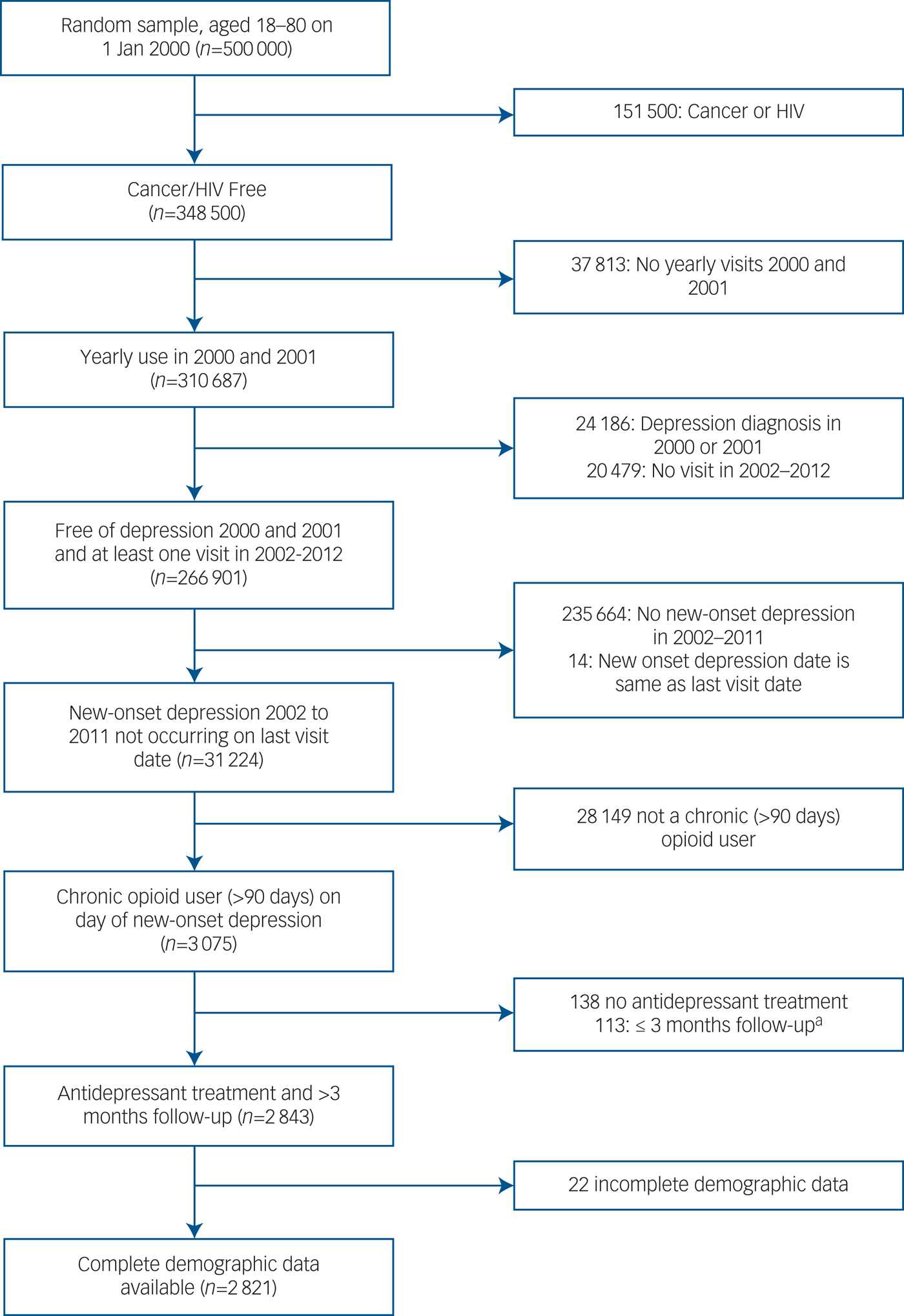
Fig. 1 Cohort selection.
Outcome – opioid cessation
Opioids included codeine, fentanyl, hydrocodone, hydromorphone, levorphanol, meperidine, oxycodone, oxymorphone, morphine and pentazocine. Both short-acting and long-acting formulations were included. Opioid prescription information included days supplied, quantity (e.g. pills or liquid volume) and unit dose (mg). OAU cessation was defined as a gap of at least 182 days from the end date of the last prescription.Reference Martin, Fan, Edlund, Devries, Brennan-Braden and Sullivan5 OAU cessation date was the first day of this gap.
Exposure – ADM adherence
ADMs included monoamine oxidase inhibitors (MAOIs), selective serotonin reuptake inhibitors (SSRIs), serotonin–norepinephrine reuptake inhibitors (SNRIs), tricyclics (TCAs) and non-classified ADMs. ADM adherence was defined using proportion of days covered (PDC) from NDE to opioid cessation or censor date.Reference Hess, Raebel, Conner and Malone19, Reference Cramer, Roy, Burrell, Fairchild, Fuldeore and Ollendorf20 ADM prescriptions dispensed were used to create time arrays to identify days of follow-up that an ADM was available. If multiple ADMs were available in a day, that particular day was only counted once as a covered day. The PDC was calculated by taking the total number of covered days in follow-up by the total number of days in follow-up. PDC was dichotomised to standard thresholds for adherence (≥80%) and non-adherence (<80%).Reference Hess, Raebel, Conner and Malone19–Reference Nau21 To determine if patient adherence was correlated with duration of ADM treatment, we computed the number of continuous weeks of treatment. ADM use was considered continuous if there was no gap of >30 days between prescriptions dispensed and duration for all periods of continuous use in follow-up were assessed to categorise duration as ever ≥24 weeks, 12 to <23 weeks or <12 weeks.
Covariates
We included an OAU duration variable to control for duration of use at the date of NDE (3–6 months, >6 to 12 months, >12 to 24 months, >24 months). Duration was computed from the months of continuous OAU (no gap >30 days between prescriptions dispensed). The opioid morphine equivalent dose (MED) was calculated using standard conversion tables. Days supplied and quantity variables were used to calculate daily MED in follow-up. We modelled the maximum daily MED before the end of follow-up (1–50 mg, 51–100 mg, >100 mg). We controlled for comedication with benzodiazepines, which are associated with long-term opioid use,Reference Quinn, Hur, Chang, Krebs, Bair and Scott22 and muscle relaxants, which could improve pain and functioning. Benzodiazepines included alprazolam, clonazepam, diazepam, lorazepam, chlordiazepoxide and clorazepate. Muscle relaxants included carisoprodol, cyclobenzaprine, baclofen, dantrolene, metaxalone, methocarbamol, chlorzoxazone, tizanidine and orphenadrine. Demographic variables included age, gender, ethnicity (white v. other), marital status (married v. other) and insurance coverage (Veterans Health Administration only v. other sources).
To control for detection bias related to more healthcare encounters, we created a healthcare utilisation variable defined as average number of out-patient clinic visits per month in follow-up. The distribution of the mean was then dichotomised into high utiliser, >75th percentile, v. low utiliser, ≤75th percentile. We controlled for psychiatric and physical comorbidities associated with depressionReference Thakore23 and/or OAU.Reference Seal, Shi, Cohen, Cohen, Maguen and Krebs24–Reference O'Rourke and Wosnitzer26 Comorbidities were defined using ICD-9-CM diagnostic codes. Psychiatric comorbidities included post-traumatic stress disorder and any other anxiety disorder, a composite of panic disorder, generalised anxiety disorder, social phobia, obsessive–compulsive disorder and anxiety disorder not otherwise specified. We controlled for alcohol misuse or dependence; illicit drug misuse or dependence, including opioids; and nicotine dependence. Chronic physical conditions included type 2 diabetes mellitus, hypertension, cerebrovascular disease, obesity, low testosterone, sleep apnoea and cardiovascular disease. Cardiovascular disease was a composite of hyperlipidaemia, ischaemic heart disease, disease of pulmonary circulation, other heart disease, hypertensive heart disease and myocardial infarction.
Five separate pain condition variables were created based on over 900 ICD-9-CM codes.Reference Scherrer, Svrakic, Freedland, Chrusciel, Balasubramanian and Bucholz7, Reference Seal, Shi, Cohen, Cohen, Maguen and Krebs24 These conditions were arthritis, back pain, musculoskeletal pain, headaches and neuropathic pain. Pain scores, collected during routine care in the Veterans Health Administration, were on a numerical rating scale ranging from 0 to 10, with higher scores indicating greater pain intensity. In propensity score models, we adjusted for a time invariant maximum pain score before the end of follow-up to control for the highest pain level. As variability in pain scores in the Veterans Health Administration have been previously reported,Reference Dobscha, Morasco, Kovas, Peters, Hart and McFarland27 a time-varying pain score for each month of follow-up was used in final survival models. For the time-varying pain score assessment, the pain score was assumed to be consistent across subsequent months until a new monthly assessment was available.
Propensity scores and inverse probability of treatment weighting (IPTW) were used to balance potential confounders listed in Table 1 between ADM adherent and non-adherent treatment groups to reduce the effect of bias by indication and other sources of confounding. The propensity score is the probability of ADM adherence, given covariates and was calculated using a binary logistic regression model. Propensity scores were used to apply IPTW approaches using stabilised weights.Reference Cole and Hernan28–Reference Xu, Ross, Raebel, Shetterly, Blanchette and Smith33 A stabilised weight is the marginal probability of ADM adherence divided by the propensity score for the adherent group, and (1 – marginal probability of ADM adherence) divided by (1 – propensity score) for the non-adherent group. It helps reduce bias associated with extreme weights of either individuals in the ADM adherent group with low propensity scores or those in the non-adherent group with high propensity scores. Extreme weights are associated with increased variability of the exposure effect, thus, stabilising weights helps reduce type II error rate.Reference Austin and Stuart34 Stabilised IPTW also preserves original sample size (i.e. does not inflate sample size in pseudo-data) in analysis thereby also preserving the type I error rate.Reference Xu, Ross, Raebel, Shetterly, Blanchette and Smith33 Stabilised weights were trimmed if they were ≥10, as well-behaved weights have a mean close to 1 and a maximum <10.Reference Harder, Stuart and Anthony30, Reference Sturmer, Wyss, Glynn and Brookhart35 The mean of stabilised weights should be close to 1, extreme values indicate the propensity-score model poorly specified predictors of treatment exposure. IPTW resulted in pseudo-populations for ADM adherence groups such that covariates balanced across the two groups. Covariate balance was assessed by comparing covariate distributions between ADM adherence groups. Balance is indicated with no significant differences in the distribution of covariates between groups and by standard mean differences <10%.Reference Austin and Stuart34, Reference Austin36
Table 1 Characteristics of patients with long-term opioid use with a new depression episode (NDE) by antidepressant medication (ADM) adherence status in unweighted data, 2002–2012 (n = 2821)

Results in bold are significant.
SMD %, standardised mean difference per cent.
a. Other anxiety disorders: panic disorder, obsessive–compulsive disorder, social phobia, generalised anxiety disorder, anxiety not otherwise specified.
b. Cardiovascular disease, hyperlipidaemia, ischaemic heart disease, diseases of pulmonary circulation, other heart disease, hypertensive heart disease, myocardial infarction.
Statistical analysis
Analyses were performed with SAS v9.4 at an alpha of 0.05. Bivariate analyses, using independent samples t-tests for continuous variables and chi-square tests for categorical variables, assessed the relationship of covariates with ADM adherence in unweighted and weighted data. A Poisson regression model was used in unweighted and weighted data to compare opioid cessation incidence rate (person-years) between ADM adherence groups. Unweighted and weighted Cox proportional hazards models were used to calculate hazard ratios and 95% confidence intervals for the relationship of ADM adherence and time to opioid cessation. Weighted analyses used stabilised weights in probability weighting. Confidence intervals and P-values in weighted analyses were calculated using robust, sandwich-type variance estimators. Follow-up time was defined as months from date of NDE to date of OAU cessation or censor date, which was the last available Veterans Health Administration encounter.
The final model included variables to control for pain diagnoses and changing pain score after the initiation of ADM treatment. All pain variables and ADM adherence were modelled as time dependent. This allows ascertainment of exposure status over the multiyear observation period and permits new diagnoses and change in pain scores to contribute to the outcome. Initial evaluation of each interaction term of each covariate and follow-up time confirmed that the proportional hazards assumption was met for ADM adherence (P = 0.11) and pain covariates (P > 0.05).
This project was approved by the institutional review boards of participating institutions.
Exploratory analysis
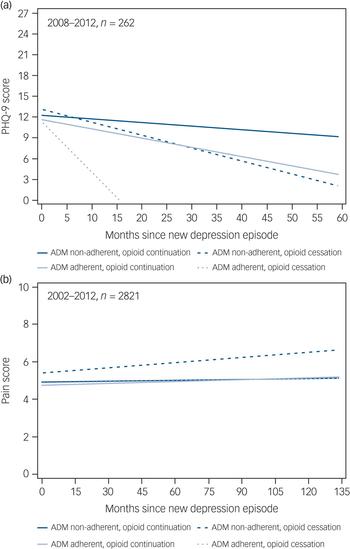
In unweighted data, the average monthly change in PHQ-9 score and pain score across follow-up was computed using random intercept longitudinal mixed models (Proc Mixed, SAS v9.4) for four groups; (a) ADM adherent with OAU cessation (n = 5 PHQ-9; n = 213 pain scores), (b) ADM adherent without OAU cessation (n = 96 PHQ-9; n = 864 pain scores), (c) ADM non-adherent with OAU cessation (n = 14 PHQ-9; n = 354) and (d) ADM non-adherent without OAU cessation(n = 147 PHQ-9; n = 1390 pain scores). Because of the lack of PHQ-9 data prior to 2008, monthly changes in PHQ-9 scores in follow-up were computed for a subset of 262 patients with NDE occurring in 2008–2012 and with at least one PHQ-9 score before end of follow-up. Time was modelled as months since NDE and models included all available pain and PHQ-9 data in follow-up.
Results
In unweighted data, a Fisher's exact test of independence revealed ADM adherence and duration were highly related (P < 0.0001, results not shown). Among 1077 patients who were adherent, 0.2% received continuous ADM for less than 12 weeks, 4.4% received an ADM for 12 to <24 weeks and 95.5% for at least 24 weeks. Among 1744 patients who were non-adherent, 15.0% received ADM for less than 12 weeks, 19.0% for 12 to <24 weeks and 66.1% for at least 24 weeks.
Figure 2 shows that the overall unweighted incidence rate for OAU cessation was 48.4 per 1000 person-years, with no significant differences between ADM adherent (50.2/1000 person-years) and non-adherent (47.4/1000 person-years) groups (P = 0.496). However, after weighting data using IPTW techniques, the incidence rate of OAU cessation was higher for ADM adherent (57.2/1000 person-years) compared with non-adherent (45.0/1000 person-years ) groups (P = 0.007).
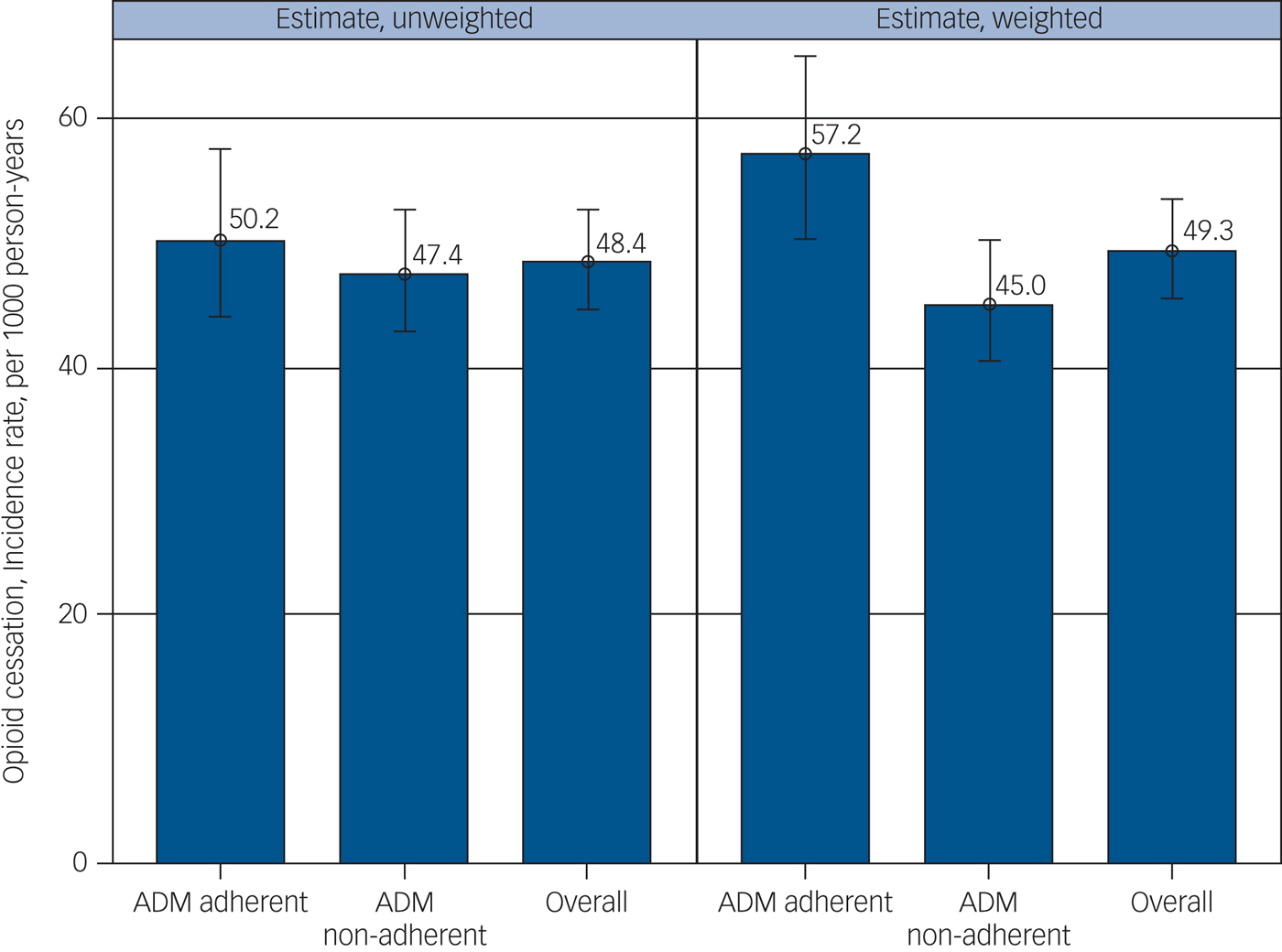
Fig. 2 Antidepressant adherence, non-adherence and prescription incidence of opioids.
Unweighted distributions of covariates by ADM adherence are shown in Table 1. Among those individuals taking opioids for >90 day with a NDE receiving ADM treatment, almost half (46.9%) had taken opioids for more than 2 years (>24 months) at the time of the NDE. Almost two-thirds (63.0%) reached a maximum MED of >100 mg. Maximum dose achieved was similar (P = 0.704) in ADM adherent and non-adherent groups. Benzodiazepine comedication with ADM was significantly more prevalent among ADM adherent (59.0%) v. non-adherent (53.6%) groups. Comorbidities that were significantly more prevalent among ADM adherent compared with non-adherent groups were post-traumatic stress disorder, type 2 diabetes, hypertension, cardiovascular disease, low testosterone and sleep apnoea. Alcohol and illicit drug misuse/dependence were more prevalent among the non-adherent group. Patients in the ADM adherent compared with the non-adherent group were significantly older, White, married, more likely to have other insurance in addition to Veterans Health Administration insurance and have higher healthcare utilisation.
After applying IPTW, all covariates balanced and were not significantly different between the ADM adherent and non-adherent groups (Table 2). IPTW stabilised weights ranged from 0.54 to 3.15, with a mean of 1.00 (s.d. = 0.25) and median of 0.95 (interquartile range (IQR) = 0.83–1.11). The standardised mean differences (SMDs) after weighting were all <10%. Good balance was achieved given differences between treatment groups were all non-significant and all SMDs were <10%.
Table 2 Weighted association of covariates with antidepressant medication (ADM) treatment adherence, weighted by inverse probability of ADM treatment adherence, in patients with chronic opioid use (>90 days) at time of new depression episode (NDE, 2002–2012; n = 2821)

SMD %, standardised mean difference per cent.
a. Other anxiety disorders: panic disorder, obsessive–compulsive disorder, social phobia, generalised anxiety disorder, anxiety not otherwise specified.
b. Cardiovascular disease, hyperlipidaemia, ischaemic heart disease, diseases of pulmonary circulation, other heart disease, hypertensive heart disease, myocardial infarction.
Results of unweighted and weighted Cox proportional hazards models estimating the association between ADM adherence and time to OAU cessation are shown in Table 3. In unweighted data, there was no relationship of ADM adherence and time to OAU cessation (hazard ratio (HR) = 1.05, 95% CI = 0.88–1.24). However, after weighting and adjusting for changes in pain scores and pain diagnoses that could occur after ADM initiation, adherence was associated with a 24% increased likelihood of OAU cessation compared with non-adherence (HR = 1.24, 95% CI = 1.05–1.46)
Table 3 Results from Cox proportional hazards models estimating the association between antidepressant medication (ADM) adherence and opioid cessation among patients with chronic opioid use ( (>90 days) with a new depression episode (NDE, 2002–2012) (n = 2821).

Results in bold are significant.
a. Unweighted data.
b. Inverse probability of adherence weighted data to control for confounding factors shown in Table 1.
c. Additional adjustment for painful conditions and pain scores after date of ADM initiation.
Longitudinal linear growth curves for PHQ-9 score among a subset of 262 patients by ADM adherent and OAU cessation groups are shown in Fig. 3(a). At time of NDE, mean PHQ-9 scores were not significantly different between groups (P = 0.995). Overall, there was a trend for a monthly decrease in PHQ-9 score (P = 0.08). Average monthly decrease in PHQ-9 scores was largest for the ADM adherent group with OAU cessation (β = −0.60, 95% CI −1.33 to 0.12) followed by the ADM non-adherent group with OAU cessation (β = −0.21, 95% CI −0.53 to 0.10), ADM adherent group without OAU cessation (β = −0.13, 95% CI = −0.21 to −0.04), and ADM non-adherent group without OAU cessation (β = −0.06, 95% CI −0.12 to 0.01). However, time trend slopes were not statistically different (P = 0.23).
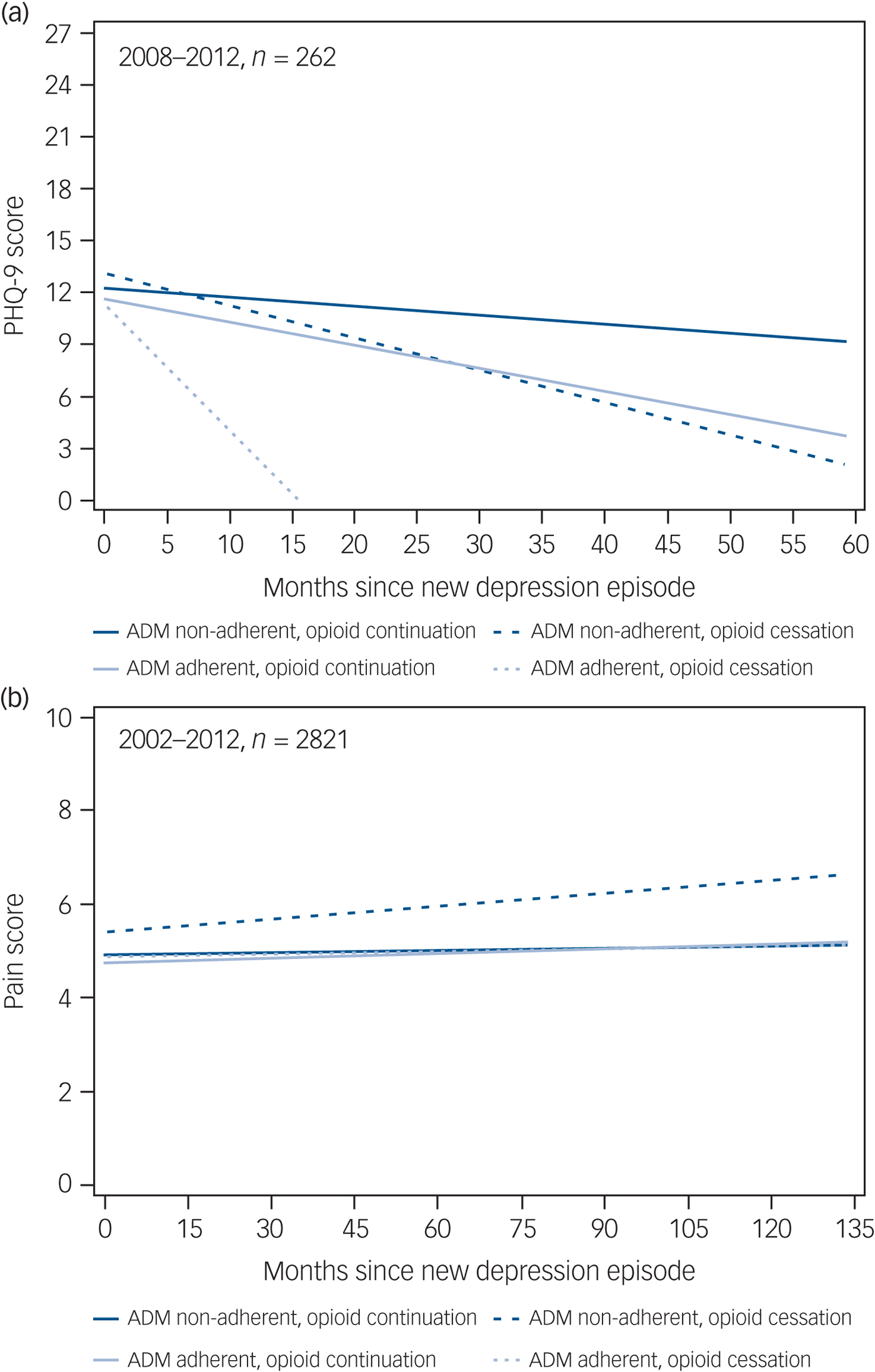
Fig. 3 Change in (a) 9-item Patient Health Questionnaire (PHQ-9) scores and (b) pain scores over time in the study groups.
Longitudinal linear growth curves for pain scores in follow-up among the entire sample of 2821 patients are shown in Fig. 3b. The ADM non-adherent group with OAU cessation had significantly higher pain scores than the other three groups at time of NDE (P = 0.0003). Results indicated there was an overall significant monthly decrease in pain score across follow-up (P = 0.002) and that these monthly changes were different between groups (P = 0.01), however, these changes were relatively flat (β range −0.0004 to 0.009).
Discussion
In a cohort of 2821 Veterans Health Administration patients who developed NDE after >90 days of prescription OAU and who received at least one ADM prescription, we observed adherence v. non-adherence to ADM treatment was associated with a 24% greater likelihood of opioid cessation. The ADM adherent v. non-adherent group had a significantly greater incidence rate of OAU cessation (57.2/1000 v. 45.0/1000 person years; P = 0.007). These results were observed after balancing factors associated with ADM. We also controlled for confounding by pain that could persist during ADM treatment.
Exploratory analysis indicates that the ADM adherent group who stopped taking opioids experienced a rapid and greater decline in depression symptoms compared with patients who did not stop taking opioids, regardless of adherence; however, these results are preliminary because cell sizes were very small for OAU cessation groups. Monthly pain scores were significantly higher among the ADM non-adherent group with OAU cessation compared with the other ADM adherent, non-adherent/opioid cessation-no cessation groups, but the size of the difference was not clinically meaningful. Adjusting for maximum pain scores after ADM initiation in the full Cox proportional hazard models did not change the association between ADM adherence and opioid cessation. Together, these results provide preliminary evidence that a reduction in depression may lead to OAU cessation. Stronger evidence indicates change in pain scores does not explain the association between ADM adherence and opioid cessation.
Interestingly, ADM adherent and non-adherent groups who stopped OAU had the steepest reductions in depression symptoms across follow-up. Because response to antidepressant treatment is markedly greater in those individuals who are adherent v. non-adherent,Reference Ho, Chong, Chaiyakunapruk, Tangiisuran and Jacob12, Reference Akerblad, Bengtsson, von Knorring and Ekselius13 we speculate people who are ADM non-adherent may have decreased PHQ-9 scores because of OAU cessation. This would be consistent with the evidence for a bidirectional relationship between OAU and depression.Reference Sullivan8 Prospective studies are warranted to verify this finding.
Patients with comorbid pain and depression may remain on opioids in an attempt to self-medicate moodReference Howe and Sullivan37 and to avoid depression during opioid withdrawal.Reference Berna, Kulich and Rathmell38 Patients with depression are more likely to drop-out of opioid taper, and withdrawal symptoms are exacerbated in patients with current depression.Reference Berna, Kulich and Rathmell38 Thus, another explanation for our findings may be related to improved depression leading to decreasing attempts to self-medicate mood and greater probability of completing opioid taper.
ADM adherence may be a proxy for overall adherence to medical treatment, the ‘healthy adherer’ effect.Reference Bitton, Choudhry, Matlin, Swanton and Shrank39 These patients may adhere to physician instructions to end OAU, adhere to other forms of pain management or begin opioid substitution treatment.
Limitations
It is possible that unmeasured confounders were not included in the propensity score and we violated the exchangeability assumption.Reference Austin and Stuart34 For instance we do not have personality measures or indicators of an orientation toward health that might predict both adherence to antidepressants and contribute to opioid cessation. Thus, unmeasured confounding is a limitation. The cohort was a majority male, Veterans Health Administration patient population, which could limit generalizability to non-Veterans Health Administration patients. However, we have previously found that the association between duration of OAU and NDEs in Veterans Health Administration patients was replicated in two private-sector cohorts,Reference Scherrer, Salas, Copeland, Stock, Ahmedani and Sullivan3 and the association between opioid use v. no use and risk of depression recurrence in Veterans Health Administration patients was replicated in a private-sector cohort.Reference Scherrer, Salas, Copeland, Stock, Schneider and Sullivan40 This suggests our findings could be replicated in private-sector cohorts. OAU was based on prescriptions dispensed and we were unable to determine whether patients took their medication as prescribed. Some OAU could be misclassified if patients transitioned to non-Veterans Health Administration or illicit sources for opioids.
Some antidepressants such as TCAs and duloxetine are used in pain management,Reference Skljarevski, Desaiah, Liu-Seifert, Zhang, Chappell and Detke41, Reference Atkinson, Patel, Meyer, Slater, Zisook and Capparelli42 but ADM management for analgesia is not designed to treat depression. Our conclusions were consistent in post hoc analysis comparing adherence to TCAs/duloxetine only v. adherence to other ADMs, indicating our findings were not as a result of adherence to only ADMs commonly used in pain management.
Implications
ADM adherence was associated with increased likelihood of OAU cessation in individuals with chronic use of opioids and this association was independent of duration of opioid use, maximum MED, pain and numerous comorbid conditions. Several studies have reported that the majority of people who take opioids for >90 days remain on opioids for 3–5 years.Reference Vanderlip, Sullivan, Edlund, Martin, Fortney and Austen4, Reference Martin, Fan, Edlund, Devries, Brennan-Braden and Sullivan5 In our cohort, 47% took them for >2 years. Thus, OAU cessation following adherent ADM treatment occurred in a patient cohort with a low probability of OAU cessation. We computed the number-needed-to-treat and found for every 20 patients adherent to ADMs, one patient will stop OAU who would not have stopped if they were non-adherent.
Treatment of opioid dependence in patients with comorbid depression may be successful following effective depression treatment. Preliminary evidence suggests OAU cessation may also contribute to improvement in depression. Therefore, opioid taper paired with ADM could result in a faster reduction of depression symptoms and increase likelihood of successful OAU cessation. Prospective data collection to obtain detailed depression and functioning measures, change in OAU and treatment trials are needed to confirm our findings.
Funding
This study was supported by the National Institute of Mental Health, Prescription Opioid Analgesics and Risk of Depression, R21MH101389. The funding sources had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The views expressed in this paper do not necessarily reflect those of the Veterans Health Administration.







eLetters
No eLetters have been published for this article.