Epidemiological studies on local or national probability samples of unrelated individuals such as those by Regier et al (Reference Regier, Farmer and Rae1990), Kessler et al (Reference Kessler, Nelson and McGonalge1996) and Grant (Reference Grant1997) have helped characterise the population distribution of alcohol dependence, its socio-demographic correlates and associations with other psychiatric disorders. Such studies have, however, largely ignored the most striking feature of alcohol dependence: its strong and apparently genetically determined familial aggregation (reviewed in McGue, Reference McGue, Zucker, Boyd and Howard1994; Reference Heath, Slutske, Madden, Wilsnack and WilsnackHeath et al, 1997b ). Positive reports are beginning to emerge from genetic linkage studies of alcohol dependence, albeit without strong replication of findings as yet (Reference Long, Knowler and HansonLong et al, 1998; Reference Reich, Edenberg and GoateReich et al, 1998), which may ultimately lead to the identification of new genetic risk or protective factors. As in other areas of psychiatry, alcoholism researchers face the challenge of developing new paradigms for ‘molecular epidemiology’, which seeks to incorporate assessment of genetic risk into epidemiological studies on general population samples.
REVIEW OF PAST PROGRESS
Aldehyde dehydrogenase gene (ALDH2)
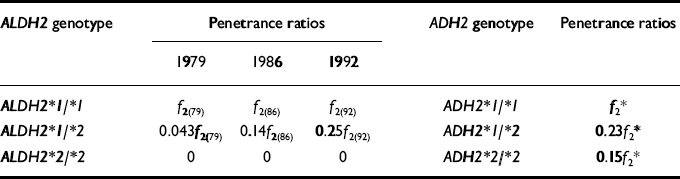
Unlike most other areas of psychiatric research, the subject of alcoholism benefits from the identification of at least two genetic polymorphisms which, in samples of Asian ancestry, have been found to be associated with differences in alcohol dependence risk. These can serve as a model system for understanding some of the challenges that will be faced in molecular epidemiological research on alcoholism. For example, Table 1 summarises ratios of the penetrances (i.e. probabilities of being an alcoholism case) of the different genotypes for two genes coding for enzymes involved in the metabolism of alcohol, aldehyde dehydrogenase (ALDH) and alcohol dehydrogenase (ADH), based on reanalyses of data on Japanese alcoholism case—control series published by Higuchi (Reference HiguchiHiguchi, 1994; Reference Higuchi, Matsushita and ImazekiHiguchi et al, 1994). Ethanol is converted by the enzyme alcohol dehydrogenase to the toxic metabolite acetaldehyde, which is in turn converted by the enzyme aldehyde dehydrogenase to acetate.
Table 1 Penetrance ratios for alcoholism in Japanese men, estimated from published data (Reference HiguchiHiguchi, 1994; Reference Higuchi, Matsushita and ImazekiHiguchi et al, 1994) on ALDH2 and ADH2 genotype frequencies in alcoholic and control series
| ALDH2 genotype | Penetrance ratios | ADH2 genotype | Penetrance ratios | ||
|---|---|---|---|---|---|
| 1979 | 1986 | 1992 | |||
| ALDH2 * 1/* 1 | f 2(79) | f 2(86) | f 2(92) | ADH2 * 1/* 1 | f 2 * |
| ALDH2 * 1/* 2 | 0.043f 2(79) | 0.14f 2(86) | 0.25f 2(92) | ADH2 * 1/* 2 | 0.23f 2 * |
| ALDH2 * 2/* 2 | 0 | 0 | 0 | ADH2 * 2/* 2 | 0.15f 2 * |
Possession of a single ALDH2*2 allele (a variant form that is rarely observed in samples of European or African—American ancestry) is associated with impaired conversion of aldehyde dehydrogenase to acetate, leading to substantially elevated blood acetaldehyde concentrations after ingestion of alcohol (Reference Yamamoto, Ueno and MizoiYamamoto et al, 1993; Reference Wall, Peterson and PetersonWall et al, 1997) and a characteristic flushing response, which is associated with a decreased risk of alcohol dependence (Reference Goedde, Agarwal and FritzeGoedde et al, 1992). Individuals who are ALDH2*2/*2 homozygotes (i.e. have two copies of the variant allele) have such an extremely adverse reaction to even moderate doses of alcohol that not a single occurrence of this genotype has been found in large series of alcoholic subjects in Japan (see Table 1; Reference Higuchi, Matsushita and ImazekiHiguchi et al, 1994; Reference Nakamura, Iwahashi and MatsuoNakamura et al, 1996; Reference Tanaka, Shiratori and YokosukaTanaka et al, 1996), China (Reference Shen, Fan and EdenbergShen et al, 1997) and Taiwan (Reference Chen, Loh and HsuChen et al, 1996). Other studies have shown that the effects of a single ALDH2*2 allele may be dependent upon drinking course: while the allele serves as a protective factor against development of alcohol problems, in those who nevertheless progress to heavier drinking, the impaired metabolism of alcoholism in turn becomes associated with increased risk of alcohol-related medical complications including alcohol-induced asthma (Reference Takao, Shimoda and KohnoTakao et al, 1998) and alcohol-related cancers (oropharyngeal, stomach, colon and lung cancers; Reference Yokoyama, Muramatsu and OhmoriYokoyama et al, 1998).
Research by Higuchi and colleagues has also demonstrated the importance of considering jointly the interplay of genetic and environmental factors. Higuchi et al (Reference Higuchi, Matsushita and Imazeki1994) have noted that the proportion of ALDH2*1/*2 heterozygotes in male alcoholic series has been increasing over time, perhaps because of recent increases in the social pressures of drinking together after work among Japanese men. We may compute from their data that the risk ratio comparing ALDH2*1/*1 homozygotes with ALDH2*1/*2 heterozygotes has declined from approximately 25:1 in 1979 to 4:1 in 1992 (see Table 1). Furthermore, while the ALDH2*1/*1 genotype is associated with increased risk, the overall probability of alcohol dependence associated with that genotype (which is shared by almost all those of European and African—American ancestry) is not great.
Higuchi et al (Reference Higuchi, Matsushita and Muramatsu1996) presented data from a small general community sample of men (n=230); only 16% of men with the ALDH2*1/*1 genotypes were estimated to have a history of alcoholism based on their questionnaire responses. The penetrance of the ALDH2*1/*1 genotype is thus quite modest, suggesting that environmental (or additional genetic) factors must be having important effects. For the same community sample, Higuchi et al (Reference Higuchi, Matsushita and Muramatsu1996) also presented data showing significant effects of ALDH2 genotype on self-reported alcohol consumption levels. From these data we may estimate that the ALDH2 locus accounts for approximately one-third of the variance in alcohol consumption in that sample. Compared with men homozygous for the low-risk ALDH2*2/*2 genotype, men homozygous for the high-risk genotype reported monthly alcohol consumption levels that were on average 10-fold higher, the corresponding difference in women being approximately 30-fold. However, the same data also point to the importance of sociocultural influences. Japanese women with the high-risk genotype were drinking at approximately the same level as men with the low-risk genotype, consistent with sociocultural restraints on drinking by Japanese women.
There is no strong tradition of psychiatric epidemiological research on general population samples in Japan. Little is therefore known about how socio-demographic factors, other environmental risk factors, or other history of psychopathology, may moderate ALDH2 genotype-specific risks of alcohol dependence. Other Asian countries, notably Korea and Taiwan (Reference Helzer, Canino and YehHelzer et al, 1990), have conducted large-scale epidemiological surveys modelled after the US Epidemiologic Catchment Area (ECA; Reference Regier, Farmer and RaeRegier et al, 1990) study, but at a time when large-scale collection of genotypic data would not have been feasible. However, a protective effect of the alcohol dehydrogenase ADH2 locus, albeit one more modest than that associated with the ALDH2*2 allele, has also been reported in individuals of Asian ancestry; and this ADH2 locus, and the closely associated ADH3 locus, are polymorphic in individuals of European and African—American ancestry (Reference Goedde, Agarwal and FritzeGoedde et al, 1992; Reference Whitfield, Nightingale and BucholzWhitfield et al, 1998) as well as in Jewish men in Israel (Reference Neumark, Friedlander and ThomassonNeumark et al, 1998).
Alcohol dehydrogenase genes (ADH2, ADH3)
Alcohol dehydrogenase, which is responsible for most of the conversion of alcohol to acetaldehyde, is formed by the combination of three different subunits (alpha, beta, gamma) encoded by closely linked loci on chromosome 4, ADH1, ADH2 and ADH3, of which only the two latter are known to be polymorphic. The low-risk ADH2*2 allele encodes the beta-2 subunit associated with faster metabolism of alcohol (and therefore a faster increase in toxic acetaldehyde levels; Reference Thomasson, Edenberg and CrabbThomasson et al, 1991) than the beta-1 subunit associated with the ADH2*1 allele (Reference Edenberg, Bosron and GuengerichEdenberg & Bosron, 1997). The ADH2*1 allele has been found to be positively associated with increased risk of alcohol dependence in Japanese people, in the work of Higuchi (Reference HiguchiHiguchi, 1994; see Table 1), as well as in Han Chinese (Reference Thomasson, Edenberg and CrabbThomasson et al, 1991; Reference Muramatsu, Zu-Cheng and Y-RuMuramatsu et al, 1995; Reference Shen, Fan and EdenbergShen et al, 1997) and Atayal Taiwanese (Reference Thomasson, Crabb and ChenThomasson et al, 1994). In the alcoholic case—control series reported by Higuchi et al (Reference Higuchi, Matsushita and Imazeki1994), in those who were ALDH2*1/*1 homozygotes, the risk of alcoholism in ADH2*1/*1 genotypes may be estimated as being approximately four times as great as that for ADH2*1/*2 heterozygotes, and six to seven times as great as that for ADH2*2/*2 homozygotes. However, from the small community series (Reference Higuchi, Matsushita and MuramatsuHiguchi et al, 1996) the risk of alcoholism in men with both risk-increasing genotypes (ADH2*1/*1 and ALDH2*1/*1 homozygotes) is only 29%.
Several reports have also stated that the ADH3*1 allele, which encodes the gamma-1 subunit associated with faster ethanol metabolism than the gamma-2 subunit encoded by ADH3*2, is associated with decreased alcohol dependence (e.g. Reference Chen, Loh and HsuChen et al, 1996; Reference Higuchi, Matsushita and MuramatsuHiguchi et al, 1996; Reference Nakamura, Iwahashi and MatsuoNakamura et al, 1996; Reference Shen, Fan and EdenbergShen et al, 1997). However, strong linkage disequilibrium (i.e. correlation between the alleles at the two loci) is observed between ADH2 and ADH3 (Reference HiguchiHiguchi, 1994), a factor that has been ignored in most analyses, and may thus cause the evidence for the protective effect of the ADH3*1 allele to be overstated. In one study which examined this possibility, the protective effect of the ADH3*1 allele was no longer observed once linkage disequilibrium between the ADH3*2 allele and the ADH2*1 allele was controlled for (Reference Osier, Pakstis and KiddOsier et al, 1999).
AIMS
The ADH2 locus is moderately polymorphic, and the ADH3 locus more highly polymorphic, in samples of populations of European ancestry (e.g. Reference Goedde, Agarwal and FritzeGoedde et al, 1992). We have published elsewhere papers presenting evidence for an important genetic contribution to alcohol dependence risk in the Australian twin panel (Reference Heath, Bucholz and MaddenHeath et al, 1997a ); the mediating role of a history of conduct problems (Reference Slutske, Heath and DinwiddieSlutske et al, 1997) and genetically determined differences in alcohol sensitivity, as assessed in an alcohol challenge paradigm (Reference Heath, Madden and BucholzHeath et al, 1999); and the absence of any major effect of genetically determined differences in blood alcohol metabolism (Reference Grant, Heath and MaddenGrant et al, 1999). We have also studied socio-demographic correlates of alcohol dependence risk, including effects of birth cohort, religious involvement and education (Reference Heath, Bucholz and MaddenHeath et al, 1997a ). A subsample of the Australian twin panel, who participated in an alcohol challenge study, have been genotyped at the ADH2 and ADH3 loci, with results generally consistent with a protective effect of the ADH2*1/*2 genotype, at least in men (Reference Whitfield, Nightingale and BucholzWhitfield et al, 1998). Here we attempt an integrated analysis of these data, which illustrates some of the challenges that must be faced in molecular epidemiological research into alcoholism.
METHOD
Sample
Characteristics of the original alcohol challenge sample (Martin et al, Reference Martin, Perl and Oakeshott1985a ,Reference Martin, Oakeshott and Gibson b and of the subsamples participating in follow-up assessments (Reference Whitfield, Nightingale and BucholzWhitfield et al, 1998; Reference Heath, Madden and BucholzHeath et al, 1999) have been described in detail elsewhere. Participants in the Australian Alcohol Challenge Twin Study (AACTS), conducted in 1979-1981, were volunteers, recruited chiefly from a volunteer national twin panel, the Australian Twin Register (ATR), which was developed with support from the Australian National Health and Medical Research Council. A total of 206 young adult twin pairs born 1944-1963 successfully completed the original alcohol challenge protocol, including 43 monozygotic (MZ) male, 45 MZ female, 37 dizygotic (DZ) male, 42 DZ female and 39 DZ unlike-sex pairs, i.e. a total of 199 men and 213 women. A mailed questionnaire survey was completed by both members of 133 complete pairs, and 16 singleton twins. An additional 3676 complete twin pairs and 551 single twins born 1893-1964, also recruited from the ATR, participated in the same mailed questionnaire survey, in 1980-1982, allowing characterisation of differences between volunteers for the alcohol challenge study and other same-age registrants with the ATR. Female AACTS participants were disproportionately heavy drinkers, whereas male participants were broadly representative of the ATR (Heath et al, Reference Heath, Madden and Martin1998, Reference Heath, Madden and Bucholz1999).
In 1992-1994 a telephone interview follow-up survey, which included assessments of history of alcohol dependence and other psychopathology (Reference Heath, Bucholz and MaddenHeath et al, 1997a ), was conducted with alcohol challenge participants and with most of the pairs who had participated in the questionnaire survey. Follow-up rates for men were 81.4% for the alcohol challenge participants (n=162), and 82.5% for other eligible males from the questionnaire sample (including 1584 men born 1944-1963); for women the rates were 87.4% for alcohol challenge participants (n=187) and 88.3% (2628 women born 1944-1963) respectively.
Assessments
Results of the alcohol challenge study (Martin et al, Reference Martin, Perl and Oakeshott1985a ,Reference Martin, Oakeshott and Gibson b ; Reference Heath and MartinHeath & Martin, 1992; Reference Whitfield, Nightingale and BucholzWhitfield et al, 1998), the 1981 questionnaire survey (Reference Kendler, Heath and MartinKendler et al, 1986) and the 1992-1994 telephone diagnostic interview follow-up assessments (Reference Heath, Bucholz and MaddenHeath et al, 1997a ) have been reported in detail. For analyses reported here, a summary alcohol sensitivity score was derived for alcohol challenge participants by principal components analysis of subjective intoxication rating and increase in static ataxia in ‘eyes open’ and ‘eyes closed’ conditions, at the first post-alcohol assessment point (Reference Heath, Madden and BucholzHeath et al, 1999). An assessment of DSM—III—R (American Psychiatric Association, 1987) alcohol dependence was adapted for telephone administration from the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Reference Bucholz, Cloninger and DinwiddieBucholz et al, 1994), and from this an approximate algorithm for DSM—IV (American Psychiatric Association, 1994) alcohol dependence could also be derived (no clustering information according to DSM—IV criteria — which did not exist at the time of the survey — was available).
Assessments of religious affiliation and of social attitudes (Reference Martin, Eaves and HeathMartin et al, 1986), used as indicators of possible population stratification effects, were included in the 1981 questionnaire survey. The ADH2 and ADH3 genotypes for 369 participants in the alcohol challenge study (176 men, 193 women) were determined, either at the time of interview follow-up, or at a separate follow-up of AACTS participants (Reference Whitfield, Nightingale and BucholzWhitfield et al, 1998), using DNA extracted from white blood cells, polymerase chain reaction (PCR) and restriction digestion, followed by electrophoresis of the PCR products (Reference von Wartburg, Gennari, Muellener, Kuriyama, Takada and Ishiivon Wartburg et al, 1988; Reference Xu, Carr and BosronXu et al, 1988). No ADH2*2/*2 homozygotes were observed in the sample.
Analyses
Alcohol dependence was analysed both as a binary variable and as a quantitative symptom count. For the latter purpose, the number of DSM—II—R alcohol dependence symptoms (e.g. tolerance, withdrawal and withdrawal relief) reported by a respondent was log transformed (ln(x+1)) prior to analysis. As a summary measure of high alcohol sensitivity, a dummy variable was created to indicate whether a participant in the alcohol challenge study had an alcohol sensitivity principal component score that fell in the upper quartile of the sensitivity distribution. Multiple regression and multiple logistic regression were used to identify predictors of dependence symptom count or diagnosis. Since there was significant evidence for linkage disequilibrium between ADH2 and ADH3 (Reference Whitfield, Nightingale and BucholzWhitfield et al, 1998), tests for ADH3 effects always included ADH2 as a covariate. Robust variance estimators were used to derive estimates of 95% confidence intervals or standard errors of parameter estimates, using STATA software (StataCorp, 1999) to adjust for the non-independence of observations on twin pairs, which would otherwise inflate estimates of statistical significance.
Where statistical tests were clearly non-significant even without adjustment for non-independence, unadjusted test statistics are reported. In some analyses, we included variables that were assessed for the entire sample (e.g. socio-demographic measures and history of psychopathology) and variables assessed only on the subsample of participants in the alcohol challenge sample (ADH2, ADH3 genotypes and the summary measure of alcohol sensitivity). A dummy variable was created indicating whether a respondent had participated in the alcohol challenge study, values for non-participants being set to zero. This allowed us to estimate effects both of variables that had been assessed for the entire sample, and of those that could only be assessed in challenge study participants.
RESULTS
ADH2, ADH3 and alcohol dependence risk
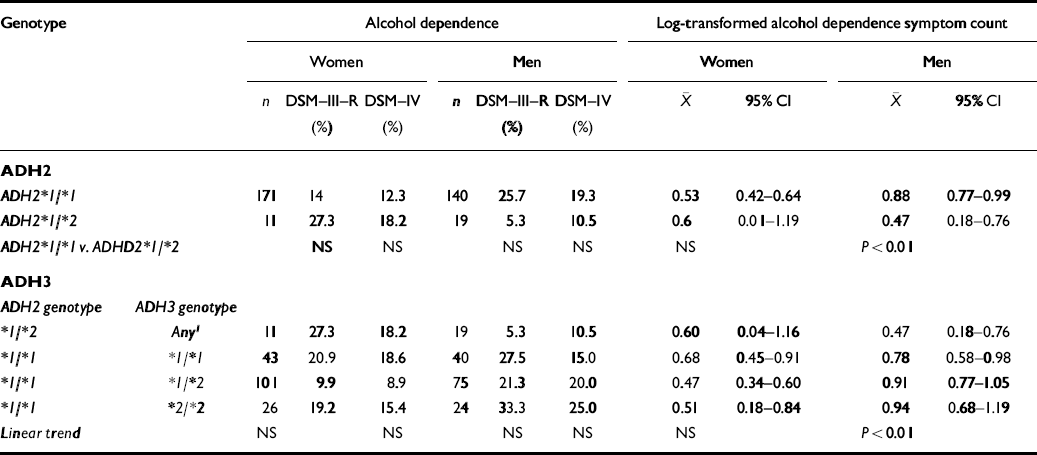
Table 2 summarises the joint association in the Australian alcohol challenge sample of ADH2 and ADH3 genotypes with a history of alcohol dependence (DSM—III—R and approximated DSM—IV) at follow-up in 1992-1994. Associations with alcohol dependence symptom count measures are also shown. Once we adjusted for the non-independence of observations on twin pairs, there was no overall association between history of alcohol dependence assessed as a binary variable and ADH2 genotype in either men or women, though there was a trend for reduced risk of DSM—III—R alcohol dependence in male ADH2*1/*2 heterozygotes, consistent with prediction (OR=0.16, 95% CI 0.02-1.23). There was, however, a significantly reduced alcohol dependence (DSM—III—R) symptom count for heterozygous men. When information on ADH3 genotype was included, no overall trend for an association of this genotype with alcohol dependence or symptom count was observed, except in the case of alcohol dependence symptom count in men. This effect was entirely explained by the protective effect of the ADH2*1/*2 genotype, with no significant evidence for an additional effect of the ADH3 genotype (unadjusted F 2,154=0.26, P=0.77).
Table 2 Associations between alcohol dehydrogenase genotypes (ADH2, ADH3) and history of alcohol dependence (DSM—III—R or DSM—IV), and DSM—III—R alcohol dependence symptom count in the Australian Twin Register
| Genotype | Alcohol dependence | Log-transformed alcohol dependence symptom count | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | ||||||||
| n | DSM—III—R (%) | DSM—IV (%) | n | DSM—III—R (%) | DSM—IV (%) | X̄ | 95% Cl | X̄ | 95% Cl | ||
| ADH2 | |||||||||||
| ADH2 * 1/* 1 | 171 | 14 | 12.3 | 140 | 25.7 | 19.3 | 0.53 | 0.42-0.64 | 0.88 | 0.77-0.99 | |
| ADH * 1/* 2 | 11 | 27.3 | 18.2 | 19 | 5.3 | 10.5 | 0.6 | 0.01-1.19 | 0.47 | 0.18-0.76 | |
| ADH2 * 1/* 1 v. ADHD2 * 1/* 2 | NS | NS | NS | NS | NS | P<0.01 | |||||
| ADH3 | |||||||||||
| ADH2 genotype | ADH3 genotype | ||||||||||
| * 1/* 2 | Any 1 | 11 | 27.3 | 18.2 | 19 | 5.3 | 10.5 | 0.60 | 0.04-1.16 | 0.47 | 0.18-0.76 |
| * 1/* 1 | * 1/* 1 | 43 | 20.9 | 18.6 | 40 | 27.5 | 15.0 | 0.68 | 0.45-0.91 | 0.78 | 0.58-0.98 |
| * 1/* 1 | * 1/* 2 | 101 | 9.9 | 8.9 | 75 | 21.3 | 20.0 | 0.47 | 0.34-0.60 | 0.91 | 0.77-1.05 |
| * 1/* 1 | * 2/* 2 | 26 | 19.2 | 15.4 | 24 | 33.3 | 25.0 | 0.51 | 0.18-0.84 | 0.94 | 0.68-1.19 |
| Linear trend | NS | NS | NS | NS | NS | p<0.01 | |||||
Predictors of alcohol dependence symptoms
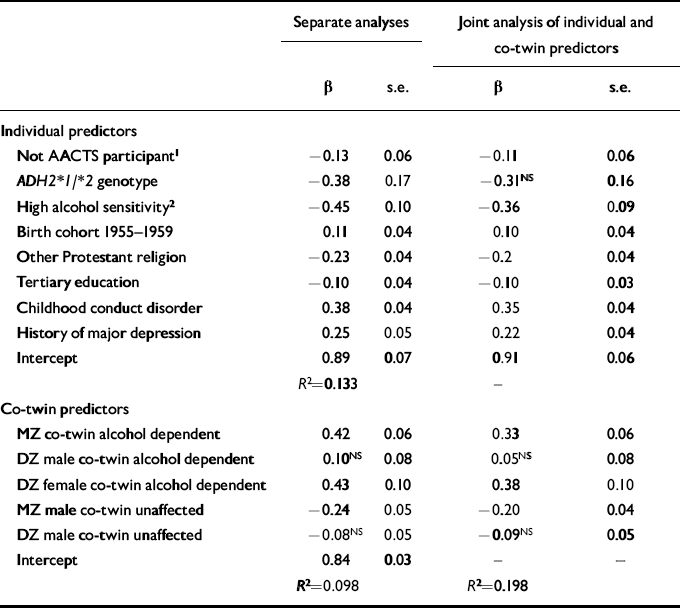
Since we found no evidence for associations between ADH2 genotype and alcohol dependence symptoms in women, subsequent analyses focused only on data from the Australian male twins. Table 3 summarises major predictors of alcohol dependence symptom count in these male twins. These data are based on all male twins born 1944-1963, with missing data on those who did not participate in the alcohol challenge study handled as described in the Method section. Columns 2 and 3 of Table 3 summarise regression coefficients from separate analyses which predicted the respondent's alcohol dependence symptom count from (a) characteristics of the respondent only, or (b) the self-report history of alcohol dependence of the respondent's co-twin, plus zygosity status of the twin pairs. Columns 4 and 5 list regression coefficients estimated under (c), a joint analysis including both sets of predictor variables.
Table 3 Results of separate and joint analyses predicting male respondents' log-transformed alcohol dependence symptom counts from own (‘individual predictor’) and co-twin characteristics. Unstandardised regression coefficient (β) and robust standard errors (s.e.) are tabulated
| Separate analyses | Joint analysis of individual and co-twin predictors | |||
|---|---|---|---|---|
| β | s.e. | β | s.e. | |
| Individual predictors | ||||
| Not AACTS participant1 | -0.13 | 0.06 | -0.11 | 0.06 |
| ADH2 * 1/* 2 genotype | -0.38 | 0.17 | -0.31NS | 0.16 |
| High alcohol sensitivity2 | -0.45 | 0.10 | -0.36 | 0.09 |
| Birth cohort 1955-1959 | 0.11 | 0.04 | 0.10 | 0.04 |
| Other Protestant religion | -0.23 | 0.04 | -0.2 | 0.04 |
| Tertiary education | -0.10 | 0.04 | -0.10 | 0.03 |
| Childhood conduct disorder | 0.38 | 0.04 | 0.35 | 0.04 |
| History of major depression | 0.25 | 0.05 | 0.22 | 0.04 |
| Intercept | 0.89 | 0.07 | 0.91 | 0.06 |
| R 2=0.133 | - | |||
| Co-twin predictors | ||||
| MZ co-twin alcohol dependent | 0.42 | 0.06 | 0.33 | 0.06 |
| DZ male co-twin alcohol dependent | 0.10NS | 0.08 | 0.05NS | 0.08 |
| DZ female co-twin alcohol dependent | 0.43 | 0.10 | 0.38 | 0.10 |
| MZ male co-twin unaffected | -0.24 | 0.05 | -0.20 | 0.04 |
| DZ male co-twin unaffected | -0.08NS | 0.05 | -0.09NS | 0.05 |
| Intercept | 0.84 | 0.03 | - | - |
| R 2=0.098 | R 2=0.198 | |||
In the first analysis, significant protective effects of the ADH2*1/*2 genotype, as well as of high alcohol sensitivity assessed in the alcohol challenge paradigm, were observed even when socio-demographic effects on alcohol dependence symptom count were controlled for. The risk-decreasing effects of the ADH2*1/*2 genotype, and of high alcohol sensitivity, were individually similar in magnitude to the risk-increasing effect of a childhood history of conduct disorder. There were more modest risk-increasing effects associated with a history of major depression, and with being born in the years 1955-1959, while ‘other Protestant’ religious affiliation and tertiary education were each associated with decreased symptom count. Participants in the alcohol challenge study reported marginally more alcohol dependence symptoms than non-participants, although this was not a strong effect.
The second analysis confirmed our previously reported evidence for genetic effects on risk, a history of alcohol dependence in an MZ co-twin being associated with a significantly elevated symptom count, compared with a positive history in a DZ co-twin, and absence of history of alcohol dependence in an MZ co-twin being associated with a significant reduction in alcohol dependence symptom count. Finally, in the joint analysis, the association between ADH2*1/*2 genotype and reduced alcohol dependence symptom count was no longer significant, once standard errors were adjusted for the non-independence of observations on twin pairs. None the less, the estimated risk-increasing effects associated with having an alcohol-dependent MZ twin brother, or an alcohol-dependent DZ twin sister or of having a history of childhood conduct disorder, and the risk-decreasing effects of possessing an ADH2*1/*2 genotype, or of showing high alcohol sensitivity, were all comparable in magnitude.
Anglican genes or population stratification effects?
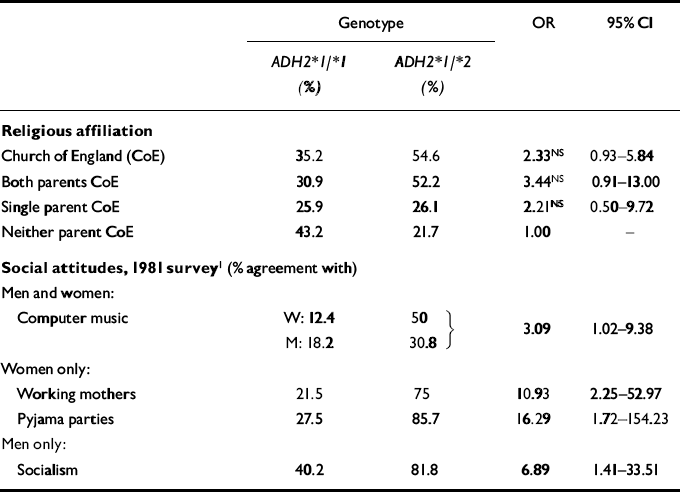
The AACTS sample provides a striking illustration of the potential dangers of ignoring population stratification effects when a traditional case—control methodology is used to incorporate collection of genetic information in epidemiological research. Table 4 summarises the associations between religious affiliation — Church of England (CoE) versus ‘Other’ — and ADH2 genotype. Although the associations fall just short of conventional significance levels, approximately one-third of those who were ADH2*1/*1 homozygotes, but 55% of those who were ADH2*1/*2 heterozygotes, reported a religious affiliation of CoE. The heterozygotes were also more likely to report that both biological parents had a CoE religious affiliation (52.2% v. 30.9%). Religious affiliation is an important correlate of a number of other socio-cultural variables. It was therefore not surprising to observe several correlations between ADH2 genotype and self-reported social attitudes (also shown in Table 4). It seems plausible that these represent associations introduced by population stratification effects, and rather less likely that a gene influencing alcohol metabolism has an indirect effect on social attitudes. Further analysis revealed, however, that religious affiliation was not associated with alcohol dependence symptom count in these data (unadjusted F 1,107=1.04, P=0.31), so that this aspect of the population stratification effect (i.e. the association between ADH2*1/*2 genotype and CoE religious affiliation) could not account for the observed association with alcohol dependence symptom count.
Table 4 Sociocultural correlates of ADH2*1/*2 genotype in the Australian Twin Register
| Genotype | OR | 95%Cl | ||
|---|---|---|---|---|
| ADH2*1/*1 (%) | ADH2*1/*2 (%) | |||
| Religious affiliation | ||||
| Church of England (CoE) | 35.2 | 54.6 | 2.33NS | 0.93-5.84 |
| Both parents CoE | 30.9 | 52.2 | 3.44NS | 0.91-13.00 |
| Single parent CoE | 25.9 | 26.1 | 2.21NS | 0.50-9.72 |
| Neither parent CoE | 43.2 | 21.7 | 1.00 | - |
| Social attitudes, 1981 survey1 (% agreement with) | ||||
| Men and women: | ||||
| Computer music | W: 12.4 | 50 [UNK] | 3.09 | 1.02-9.38 |
| M: 18.2 | 30.8 [UNK] | |||
| Women only: | ||||
| Working mothers | 21.5 | 75 | 10.93 | 2.25-52.97 |
| Pyjama parties | 27.5 | 85.7 | 16.29 | 1.72-154.23 |
| Men only: | ||||
| Socialism | 40.2 | 81.8 | 6.89 | 1.41-33.51 |
CONCLUSION
In populations of predominantly European ancestry, twin and adoption studies (reviewed by Reference McGue, Zucker, Boyd and HowardMcGue, 1994; Reference Heath, Slutske, Madden, Wilsnack and WilsnackHeath et al, 1997b ), including diagnostic interview studies on large general population samples of twin pairs (Kendler et al, Reference Kendler, Heath and Neale1992, Reference Kendler, Neale and Heath1994; Reference True, Heath and RomeisTrue et al, 1996; Reference Heath, Bucholz and MaddenHeath et al, 1997a ; Reference Prescott and KendlerPrescott & Kendler, 1999), have produced consistent evidence of a strong genetic contribution to differences in alcohol dependence risk, perhaps accounting for as much as 50-60% of the variance in risk. It is to be anticipated that current gene-mapping studies will ultimately be successful in identifying important but as yet unknown genetic risk factors for alcohol dependence. Because such gene-mapping studies typically use high-density pedigrees containing many affected family members, their findings cannot easily be generalised to the general population — a problem well illustrated in the field of breast cancer research, where a long delay occurred between identification of the risk-increasing BRCA1 gene, and characterisation of its effects in the general population (Reference CouchCouch, 1998; Reference Newman, Mu and ButlerNewman et al, 1998). This problem may be even more pronounced in the case of alcoholism, where a high proportion of cases in the community remain untreated (e.g. Reference Regier, Farmer and RaeRegier et al, 1990; Reference True, Heath and RomeisTrue et al, 1996).
It is in principle both easy and cheap to incorporate DNA collection (by blood samples or buccal scraping) in epidemiological surveys of general population samples, or in case—control series, allowing typing of candidate genes that are believed to be risk or protective factors. However, because there may be pronounced gene frequency differences among individuals of different ancestries, there is a very real danger that studies using a traditional case—control methodology, which ignores possible population stratification effects, will generate innumerable false-positive findings. This danger is especially grave for addictive behaviours such as smoking or alcohol misuse, for which there are pronounced sociocultural variations in prevalence rates. Even in the case of functional polymorphisms, population stratification effects may be important confounders, as was illustrated by the association between the protective ADH2*2 allele and an Anglican religious affiliation, and various associated social attitudes, in the Australian twin panel.
One solution to the problem of population stratification lies in targeting research efforts at population isolates such as those in Finland (e.g. Reference Hastbacka, de la Chapelle and KaitilaHastbacka et al, 1992) or Japan, which show much greater genetic homogeneity than societies such as the USA or Australia. Even within such societies, however, regional variations in patterns of substance use may be correlated with gene frequency differences, leading to the possibility of false-positive findings (Reference Kittle, Perola and PeltonenKittle et al, 1998). Furthermore, much lower rates of illicit drug problems in such societies may limit the generalisability of findings to US and other Western populations.
Use of within-family comparisons offers a much surer way to control for population stratification effects. This can be achieved most powerfully by the genotyping of both biological parents as well as the proband, in an approach popularised by Spielman and Ewens as the transmission disequilibrium test (TDT; Reference Spielman, McGinnis and EwensSpielman et al, 1993; Reference Ewens and SpielmanEwens & Spielman, 1995). In comparisons limited to heterozygous parents, the frequencies with which a candidate allele versus other alleles are transmitted to an affected offspring are compared by McNemar's test or similar matched pairs techniques. While only heterozygous parents will be informative for a TDT analysis, using dummy variables to compare disease-candidate allele associations where the candidate allele has been transmitted from heterozygous versus homozygous parents can provide a check for whether stratification effects seem to be inflating the association observed in standard case—control comparisons.
A potential concern is the availability of parents for such investigations, considering the mortality associated with alcoholism and its associated behaviours (especially smoking: Reference Hurt, Offord and CroghanHurt et al, 1996). However, this is less of an issue once it is recognised that epidemiological surveys of the origins of alcohol dependence need to focus on the period of adolescence and early adulthood. In the US National Comorbidity Survey, the median age of onset of alcohol dependence symptoms was approximately 20 years (Reference Nelson, Heath and KesslerNelson et al, 1998), and the much larger US National Longitudinal Alcohol Epidemiologic Survey (Reference GrantGrant, 1997) likewise found very high life-time rates of alcohol dependence in the youngest age cohort. Where DNA from one or both parents is unavailable, comparison of unaffected and affected full siblings permits an equivalent test, comparing allele counts or genotype frequencies in discordant sib pairs (Reference Spielman and EwensSpielman & Ewens, 1998). Using discordant sib pairs is much less powerful than using case—parent trios; however, families with at least two affected and one unaffected siblings are particularly informative, and for high prevalence traits can compare favourably with the standard trios (Reference Hovarth and LairdHovarth & Laird, 1998). Unfortunately, given the small sample sizes and quasi-random sampling used in the AACTS, we had insufficient power to use such methods to confirm the association that we observed between ADH2 and reduced alcohol dependence symptom count in men.
The growing understanding of the role of genetic factors in the aetiology of alcohol dependence has important clinical implications. There is no simple ‘one gene—one disease’ relationship, but rather a multiplicity of genetic factors (including, in Asians, ALDH2 and ADH2 as well as possibly ADH3 variants) that contribute, in conjunction with environmental risk factors, to differences in alcohol dependence risk. Already, for alcoholic patients of Asian origin, a case could be made for determining ALDH2 genotype, since ALDH2*1/*2 heterozygotes who develop problems with alcohol appear at heightened risk of medical complications. With the growth of transracial adoptions, an increasing number of children of Asian ancestry are being reared by parents of European ancestry, whose physicians are less likely to be aware of such relationships between genotype, alcohol exposure and risk of medical complications of alcohol dependence.
The Australian data that we have used to illustrate our discussion are of course not without limitations. The Australian twin panel was developed as a volunteer panel through appeals in the media, and while a broad range of socio-economic levels and educational backgrounds are represented in the sample (Reference Heath, Slutske, Madden, Wilsnack and WilsnackHeath et al, 1997b ), it cannot be considered to be truly representative of the population of Australia. Genotyping at the ADH2 and ADH3 loci has as yet been completed only for participants in the alcohol challenge study and some of their relatives, so that failure to detect significant protective effects of the ADH2 locus in women may be a consequence of low power. Furthermore, we know that women who volunteered to participate in the alcohol challenge study were disproportionately heavy drinkers (Reference Heath, Madden and MartinHeath et al, 1998), and thus we might expect to find that ADH2*1/*2 heterozygotes were underrepresented among these volunteers; there is indeed some evidence to this effect, a higher frequency of the heterozygotes being observed in men than in women (Reference Whitfield, Nightingale and BucholzWhitfield et al, 1998). Notwithstanding these limitations, the Australian data provide a useful example both of the possibilities of studying jointly the effects of genetic and environmental risk factors, and of the potential confounding factors that may arise.







eLetters
No eLetters have been published for this article.