Handedness refers to the preference for, or the use of, one hand over the other hand, and can already be observed on ultrasound scans early in prenatal life (Hepper, Reference Hepper2013; Hepper et al., Reference Hepper, Shahidullah and White1991; Parma et al., Reference Parma, Brasselet, Zoia, Bulgheroni and Castiello2017). The most common form of handedness is right-handedness (RH), with a prevalence of around 90% in nearly all human populations, and the remaining is non-right-handed, which subsumes left- and mixed-handedness (LH and MH). Comparing twins with singletons in large meta-analysis, the prevalence of non-right-handedness (NRH) is higher in twins (11.11%) than in singletons (7.23%) (Pfeifer et al., Reference Pfeifer, Schmitz, Papadatou-Pastou, Peterburs, Paracchini and Ocklenburg2022), and it has been hypothesized that this NRH is related to a higher rate of complications during multiple pregnancies and births (Davis & Annett, Reference Davis and Annett1994; de Kovel et al., Reference de Kovel, Carrión-Castillo and Francks2019; Ellis et al., Reference Ellis, Ellis and Marshall1988; Heikkilä et al., Reference Heikkilä, van Beijsterveldt, Haukka, Iivanainen, Saari-Kemppainen, Silventoinen, Boomsma, Yokoyama and Vuoksimaa2018; Heikkilä et al., Reference Heikkilä, Vuoksimaa, Saari-Kemppainen, Kaprio, Rose, Haukka, Pitkäniemi and Iivanainen2015; Pfeifer et al., Reference Pfeifer, Schmitz, Papadatou-Pastou, Peterburs, Paracchini and Ocklenburg2022; Sicotte et al., Reference Sicotte, Woods and Mazziotta1999; Vuoksimaa et al., Reference Vuoksimaa, Koskenvuo, Rose and Kaprio2009). Twins are generally born preterm and have a lower birth weight than singletons. They are also at a greater risk of congenital disorders and perinatal morbidity (Willemsen et al., Reference Willemsen, Odintsova, de Geus, Boomsma, Khalil, Lewi and Lopriore2021). The increased risk of complications in twins may be related to increased NRH, but a within-family analysis of twins and their siblings showed that the prevalence of LH among twins was comparable to that among their non-twin siblings (Medland et al., Reference Medland, Duffy, Wright, Geffen, Hay, Levy, van-Beijsterveldt, Willemsen, Townsend, White, Hewitt, Mackey, Bailey, Slutske, Nyholt, Treloar, Martin and Boomsma2009; Medland et al., Reference Medland, Wright, Geffen, Hay, Levy, Martin and Duffy2003).
Hypotheses concerning the etiology of handedness include polygenic (Armour et al., Reference Armour, Davison and McManus2014) and epigenetic factors (Odintsova et al., Reference Odintsova, Suderman, Hagenbeek, Caramaschi, Hottenga, Pool, Consortium, Dolan, Ligthart, van Beijsterveldt, Willemsen, de Geus, Beck, Ehli, Cuellar-Partida, Evans, Medland, Relton, Boomsma and van Dongen2022), environmental influences, and random or stochastic events (Annett, Reference Annett1985; I. McManus, Reference McManus1985). Medland et al. (Reference Medland, Duffy, Wright, Geffen, Hay, Levy, van-Beijsterveldt, Willemsen, Townsend, White, Hewitt, Mackey, Bailey, Slutske, Nyholt, Treloar, Martin and Boomsma2009) studied 54,270 twins and their non-twin siblings. Correcting for year of birth, birth weight and sex, the heritability of LH was estimated to be around 24%, with the remainder accounted for by nonshared environment. The heritability estimate based on measured genetic variants was 3.45% in a meta-analysis of genetic association studies (Cuellar-Partida et al., Reference Cuellar-Partida, Tung, Eriksson, Albrecht, Aliev, Andreassen, Barroso, Beckmann, Boks, Boomsma, Boyd, Breteler, Campbell, Chasman, Cherkas, Davies, de Geus, Deary, Deloukas and Medland2021) that found 41 loci associated with LH. A recent meta-analysis of DNA methylation in two cohorts (N bloodDNA = 3914, N buccal = 1737) detected a few cohort-specific differentially methylated regions associated with LH, but no robustly associated DNA methylation loci (Odintsova et al., Reference Odintsova, Suderman, Hagenbeek, Caramaschi, Hottenga, Pool, Consortium, Dolan, Ligthart, van Beijsterveldt, Willemsen, de Geus, Beck, Ehli, Cuellar-Partida, Evans, Medland, Relton, Boomsma and van Dongen2022).
Identification of environmental factors is likely to be difficult (C. McManus, Reference McManus2021), and the associations with pre- and perinatal factors were a focus for several decades. Bakan et al. (Reference Bakan, Dibb and Reed1973) related NRH to the ‘birth stress’ index, which incorporated multiple births, premature birth, prolonged labor, cesarean section, breech position and breathing difficulty at birth; that is, risk factors that may cause perinatal hypoxia and brain damage.
A meta-analysis of 23 studies comprising ∼47,000 individuals reported that prenatal stress, fetal presentation (breech position in males), gestational age and mode of delivery (cesarean section) were associated with NRH (Searleman et al., Reference Searleman, Porac and Coren1989). A Danish study of ∼35,000 singletons found associations between MH and mother’s and father’s handedness, preterm birth and mode of conception with children conceived after intrauterine insemination having twice the risk of being mixed-handed compared to naturally conceived children (Zhu et al., Reference Zhu, Obel, Basso, Bech, Henriksen and Olsen2009). Associations with parental age were inconsistent (Bailey & McKeever, Reference Bailey and McKeever2004; Dragović et al., Reference Dragović, Milenković, Kocijancić and Zlatko2013; Johnston et al., Reference Johnston, Nicholls, Shah and Shields2013; Karev, Reference Karev2008; Searleman et al., Reference Searleman, Porac and Coren1989). Stressful events in the third period of pregnancy were related to a higher prevalence of MH in offspring (Gutteling et al., Reference Gutteling, de Weerth and Buitelaar2007; Obel et al., Reference Obel, Hedegaard, Henriksen, Secher and Olsen2003). Preterm born children were more often NRH than children born after 37 weeks of gestation (Domellöf et al., Reference Domellöf, Johansson and Rönnqvist2011; van Heerwaarde et al., Reference van Heerwaarde, van der Kamp, van der Aa, de Vries, Groenendaal, Jongmans, Eijsermans, Koopman-Esseboom, van Haastert, Benders and Dudink2020). NRH was also related to low Apgar scores (Dragović et al., Reference Dragović, Milenković, Kocijancić and Zlatko2013) and adverse later outcomes, including neurodevelopment and neuropathology (Darvik et al., Reference Darvik, Lorås and Pedersen2018), and externalizing problems (Dinsdale et al., Reference Dinsdale, Reddon and Hurd2011; Logue et al., Reference Logue, Logue, Kaufmann and Belcher2015; van der Feen et al., Reference van der Feen, Zickert, Groothuis and Geuze2020). A large study of ∼500,000 adult participants from the UK Biobank reported higher rates of LH in males, increased prevalence of LH with more recent year of birth up to 1970, a higher prevalence of LH if born in the UK, if born during summer, if of lower birth weight, and not being breastfed (de Kovel et al., Reference de Kovel, Carrión-Castillo and Francks2019).
Some studies reported that first-born twins are more likely to be left-handed than second-born twins (James & Orlebeke, Reference James and Orlebeke2002), but this finding has not been replicated (Derom et al., Reference Derom, Thiery, Vlietinck, Loos and Derom1996; Medland et al., Reference Medland, Wright, Geffen, Hay, Levy, Martin and Duffy2003, Reference Medland, Duffy, Wright, Geffen, Hay, Levy, van-Beijsterveldt, Willemsen, Townsend, White, Hewitt, Mackey, Bailey, Slutske, Nyholt, Treloar, Martin and Boomsma2009; Vuoksimaa et al., Reference Vuoksimaa, Eriksson, Pulkkinen, Rose and Kaprio2010). One hypothesis is that the monozygotic twinning split could be related to NRH (Sicotte et al., Reference Sicotte, Woods and Mazziotta1999). However, monozygotic (MZ) and dizygotic (DZ) twins showed no difference in their prevalence of LH (Derom et al., Reference Derom, Thiery, Vlietinck, Loos and Derom1996; Medland et al., Reference Medland, Wright, Geffen, Hay, Levy, Martin and Duffy2003; Sicotte et al., Reference Sicotte, Woods and Mazziotta1999), and among MZ twins, no effect of chorionicity was found (Derom et al., Reference Derom, Thiery, Vlietinck, Loos and Derom1996; van Beijsterveldt et al., Reference van Beijsterveldt, Overbeek, Rozendaal, McMaster, Glasner, Bartels, Vink, Martin, Dolan and Boomsma2016).
Our overview of 32 studies on the role of early life characteristics in handedness is presented in Supplementary Table 1. Clearly, many effects do not replicate. This may be due to differences in the definitions of handedness, the study populations, differences in assessment methods, or in the definition of predictor variables and limited sample size. Pfeifer et al. (Reference Pfeifer, Schmitz, Papadatou-Pastou, Peterburs, Paracchini and Ocklenburg2022) carried out a systematic review of handedness in twins that identified 59 studies of 63,295 twins, and performed meta-analyses that revealed sources of heterogeneity related to year of publication (pre- and post-1975), availability of information on pre- and perinatal conditions that could have a moderating effect on relationship between handedness and multiple birth in different studies, differences in definition of handedness (particularly MH), and determination of zygosity.
Here we present the results of analyses in a large sample of twins. We considered three definitions of handedness: (1) right-handed versus left-handed, (2) right-handed versus mixed-handed, and (3) right-handed versus non-right-handed, aiming (a) to determine whether the risk factors for handedness, as previously identified in singleton and population-based cohorts, replicate in twins; and (b) to test the associations between handedness and twin-specific characteristics. To these ends, we analyzed data on handedness from 5-year-old twins from the Netherlands Twin Register (NTR) who were born in the Netherlands after 1986 and followed longitudinally since birth (Ligthart et al., Reference Ligthart, van Beijsterveldt, Kevenaar, de Zeeuw, van Bergen, Bruins, Pool, Helmer, van Dongen, Hottenga, van’t Ent, Dolan, Davies, Ehli, Bartels, Willemsen, de Geus and Boomsma2019).
Materials and Methods
Overview
We studied the association between handedness at 5 years and early life characteristics and outcomes in 37,495 Dutch twins. We considered three handedness definitions and investigated 23 variables related to prenatal and early life (see Supplementary Table 2). Six variables have been studied by de Kovel et al. (Reference de Kovel, Carrión-Castillo and Francks2019), and 13 have featured in previous studies of early life characteristics in association with handedness, including twin-specific characteristics such as zygosity and chorionicity. In addition, we included amnionicity and the time interval between the birth of the first and second twin, characteristics that have not been considered previously, although increased time interval has been associated with adverse neonatal outcomes for the second twin (Lindroos et al., Reference Lindroos, Elfvin, Ladfors and Wennerholm2018) and can be considered an indicator of birth-stress. As handedness is related to neurodevelopmental delays (Darvik et al., Reference Darvik, Lorås and Pedersen2018) and externalizing problems (Dinsdale et al., Reference Dinsdale, Reddon and Hurd2011; Logue et al., Reference Logue, Logue, Kaufmann and Belcher2015; van der Feen et al., Reference van der Feen, Zickert, Groothuis and Geuze2020), we included neurodevelopmental delay at 5 years, and externalizing problems at 7 years.
Subjects
Initially, we obtained data on 38,496 5-year-old twins who were born between 1989 and 2014 and were registered with the NTR by their parents at birth. Informed consent was obtained from parents prior to recruitment. Surveys, including questions on pregnancy, birth details and the 23 variables of interest were sent to mothers after registration of their newborn twins. After excluding the twins without data on handedness, the resulting sample size was 37,495 twins in 18,630 complete and 235 incomplete twin pairs, and 31,813 parents of twins. The zygosity of the same-sex twins was determined by blood/DNA polymorphisms (18%), or by parental reports based on questionnaires addressing several aspects of physical resemblance and the degree to which the twins are confused by their parents or by other relatives and strangers (Ligthart et al., Reference Ligthart, van Beijsterveldt, Kevenaar, de Zeeuw, van Bergen, Bruins, Pool, Helmer, van Dongen, Hottenga, van’t Ent, Dolan, Davies, Ehli, Bartels, Willemsen, de Geus and Boomsma2019). In 174 twins (0.46%), the information on zygosity was missing, and those were excluded from analysis testing zygosity differences.
Handedness
Handedness in twins was assessed at age 5 by the question: ‘Which hand do the children use to draw on paper?’, which was answered for both twins. We found that this item captured the relevant information on which hand was used by the child when drinking from a cup, eating, throwing a ball, picking up a coin, and combing their hair (see Supplementary Table 3). Response options were ‘left hand’, ‘right hand’, ‘both hands’ and ‘I don’t know’. Twins with missing data (n = 949) or the response ‘I don’t know’ (n = 52) were excluded from the study. Parents’ handedness was obtained from the survey collected when the twins were 2 years (Orlebeke et al., Reference Orlebeke, Knol, Koopmans, Boomsma and Bleker1996), which included a question about mother’s and father’s hand preference with three response categories: left-handed, right-handed and both hands. For current analysis, LH was coded as right-handed (0) and left-handed (1). MH was coded as right-handed (0) and mixed-handed (1). NRH was coded as right-handed (0) and non-right-handed (left-handed and mixed-handed combined) (1).
Early Life Characteristics
We investigated the following five sets of early life characteristics (see Supplementary Table 2):
(1) General: demographic (sex, year of birth), familial (mother’s and father’s handedness).
(2) Prenatal: mother’s and father’s age at birth, mode of conception (natural vs. assisted), prenatal maternal smoking (no/yes), and maternal stress during pregnancy (no/yes).
(3) Perinatal: season of birth (being born in summer or not), gestational age (continuous), fetal presentation at birth (cephalic presentation and noncephalic presentation: breech and horizontal), mode of delivery (vaginal and intervention with vacuum extraction, forceps, or cesarean section), birth weight (continuous), and Apgar score at 1st minute (continuous score).
(4) Postnatal: breastfeeding (no/yes), neurodevelopmental delay (no/yes), and externalizing problems (continuous score). Neurodevelopmental delay was assessed based on bowel and bladder toilet skill delay at age five by the questions ‘How often do the children defecate in their pants?’ and ‘How often do the children pee in their pants during the day?’ (see Supplementary Appendix 1). Normal development in bowel and bladder toilet skills is indicated by having fewer than four wetting accidents per week by the age of 4 (Francis et al., Reference Francis, Mannion and Leader2017; Schum et al., Reference Schum, Kolb, McAuliffe, Simms, Underhill and Lewis2002). Externalizing problems were assessed using the subscale of the Aggressive Behavior of the Child Behavior Checklist (CBCL), which mothers completed when their children were 7 years old (Achenbach & Rescorla, Reference Achenbach and Rescorla2001).
(5) Twin-specific: zygosity (dizygotic or monozygotic), chorionicity (dichorionic or monochorionic), amnionicity (diamniotic or monoamniotic), birth order (first or second born), and the time interval between the birth of the first- and second-born twin.
Pre- and perinatal characteristics were obtained from the survey that was sent to mothers in the first year after the birth of the twins. Parents’ handedness and information on breastfeeding were obtained from the survey collected when the twins were 2 years (Orlebeke et al., Reference Orlebeke, Knol, Koopmans, Boomsma and Bleker1996), which included a question about mother’s and father’s hand preference with three response categories: left-, right-handed, and both hands, and the question: ‘Was your twin breastfed?’ (responses categorized into yes/no responses). Information on toilet skills was obtained from the survey at 5 years. Information on chorion status was obtained by linking to the records from the database of the Dutch pathological anatomy national automated archive (PALGA; van Beijsterveldt et al., Reference van Beijsterveldt, Overbeek, Rozendaal, McMaster, Glasner, Bartels, Vink, Martin, Dolan and Boomsma2016). The distributions of the data, broken down by the definition of handedness, are presented in Supplementary Figures 1 and 2. Several variables were characterized by missingness due to the absence of questions in earlier surveys, nonresponse, or absence in the record linkage: chorionicity and amnionicity (70% missing), fetal presentation at birth (81%), maternal stress during pregnancy (81%), Apgar scores at 1 minute (86%), and aggression at age 7 (45%).
Continuous variables were dichotomized to report odd ratios. To dichotomize year of birth the mean (born in 2000) was used. To dichotomize parental age the value of 40 years was chosen. For gestational age, the sample was divided in full-term birth (>37 weeks) and preterm birth (<37 weeks), and the additional sensitivity analysis was performed with this categorical variable. Birth weight was categorized at 2500 g. The time interval between the twin births included six cases with extreme values (i.e., more than 33 hours between birth of first- and second-born twins). These extreme values were recoded as missing. The time between birth of first and second twins was divided into two groups <30 minutes and >30 minutes for dichotomous variable. Apgar score was divided into two groups below and above 7 points that corresponds to lower threshold of intermediate values of the scale (Odintsova, Dolan et al., Reference Odintsova, Dolan, van Beijsterveldt, de Zeeuw, van Dongen and Boomsma2019). For aggression, a sum score defined high-scoring children based on mother rating ≥5 points, the same as detailed in Hagenbeek et al. (Reference Hagenbeek, Roetman, Pool, Kluft, Harms, van Dongen, Colins, Talens, van Beijsterveldt, Vandenbosch, de Zeeuw, Déjean, Fanos, Ehli, Davies, Hottenga, Hankemeier, Bartels, Vermeiren and Boomsma2020).
Statistical Analysis
All statistical analyses were performed in R version 4.1.0. Due to missingness in early life characteristics, the number of subjects in the regression analyses varied between 4515 and 37,495.
Descriptive Statistics
The frequencies of categorical variables, means and standard deviations (SD) of the continuous variables were obtained in the total sample, and within the RH, LH and MH groups. As descriptive measures of association between early life characteristics, we report Pearson correlations for the continuous variables (R package ‘stats’), polychoric correlations for the categorical variables (R package ‘polychor’) and point polyserial correlations between the continuous and categorical variables (R package ‘stats’). The differences in proportion of LH/MH/NRH versus right-handed twins in a previously published meta-analysis (Pfeifer et al., Reference Pfeifer, Schmitz, Papadatou-Pastou, Peterburs, Paracchini and Ocklenburg2022) and the current study sample, between males and females, in NRH parents, in twins from the same-sex and opposite-sex pairs, in preterm and full-term born were tested with the two-sample test for equality of proportions (R package ‘stats’).The same test was applied to test differences in proportion of LH/MH/NRH fathers and mothers.
Association Analysis
Given dichotomous predictors and dichotomized continuous predictors, we reported the odds ratios (ORs) and the 95% confidence intervals (CI) (R package ‘epitools’). An OR of 1 indicates no group differences, ORs > 1 suggest a higher prevalence of LH, MH or NRH in categories of early life characteristics, and ORs < 1 suggest a higher prevalence of RH. For testing associations between handedness and the characteristics of interest, we used generalized estimating equation (GEE) regression analyses to account for the relatedness of the twins. We conducted three GEE analyses in which we related the 23 predictors to the three definitions of handedness. The coding of the predictors is presented in Supplementary Table 2.
Explained Variance
To determine the amount of variance in handedness explained by early life factors, the model was fitted with logistic regression, including seven characteristics that were significant in the association analysis. The model was applied to all three definitions of handedness. The explained variance explained by the seven characteristics was obtained on the liability scaled underlying binary responses as proposed by Lee et al. (Reference Lee, Goddard, Wray and Visscher2012).
Multiple Testing Corrections
As a Bonferroni correction for multiple testing tends to be conservative, we considered the correction suggested by Nyholt (Reference Nyholt2004), which involves calculating the effective number of tests given the correlations among the predictors. Correlations among the 23 early life characteristics are presented in Supplementary Figure 3. The mean correlation was .14, and the effective number of tests was 22.5 (see Supplementary Appendix 2). Given the two definitions (LH and MH; note that NRH represents the combination of these two), we set the alpha-per-test to equal α = 0.05/(22.5*2) = 0.001.
Ethics Statement
The study protocols were approved by the Central Ethics Committee on Research Involving Human Subjects of the VU University Medical Center, Amsterdam). A written informed consent was given by parents of participants for the children and themselves.
Data Availability
Data were provided by the Netherlands Twin Register (reference NTR-DSR-2552) and can be requested for replication or alternative analyses through the NTR data access procedure (https://tweelingenregister.vu.nl/information_for_researchers/working-with-ntr-data).
Results
Descriptives: Prevalence of Handedness in Twin Families
Twins
The study cohort included 37,495 twins with measurements of handedness at 5 years old from 18,865 twin pairs born between 1986 and 2014. Of these, 49.7% were males and 35% were MZ twins. Characteristics of the participants are given in Table 1. The prevalence of RH was 83.81% and NRH was 16.19% (14.86% LH and 1.33% MH). The prevalence of LH in twins was higher than the prevalence in adult singletons, as reported in a recent meta-analysis (14.86% in our study vs. 6.97% in singletons; Pfeifer et al., Reference Pfeifer, Schmitz, Papadatou-Pastou, Peterburs, Paracchini and Ocklenburg2022), χ2(1) = 2326.5, p <0.001). The prevalence of MH in twins was lower than the prevalence in adult singletons (1.33% in our study vs. 2.67% in singletons; Pfeifer et al., Reference Pfeifer, Schmitz, Papadatou-Pastou, Peterburs, Paracchini and Ocklenburg2022), χ2[1] = 102.68, p < .001). The prevalence of LH and MH was higher in boys than in girls (15.9% of left-handed boys vs. 13.8% of left-handed girls, χ2[1] = 32.74, p < .001; and 1.8% of mixed-handed boys vs. 0.8% of mixed-handed girls, χ2[1] = 66.3, p < .001).
Table 1. Characteristics of twin participants

Note: LH, left-handed; RH, right-handed; MH, mixed-handed. Values are presented as mean and standard deviation (SD) or number (n) and frequency in the group (%).
Parents of twins
In the NTR, 84.91% of the parental generation with known handedness (n = 31,813, 49.9% males) were right-handed and 15.09% were non-right-handed, of whom 11.45% were left-handed, and 3.64% were mixed-handed. Thus, the prevalence of NRH was higher in parents of twins than in adult singletons (15.09% vs. 7.23%; Pfeifer et al., Reference Pfeifer, Schmitz, Papadatou-Pastou, Peterburs, Paracchini and Ocklenburg2022), χ2[1] = 2010.3, p < .001). The prevalence of LH was higher in fathers than in mothers (12.4% of left-handed fathers vs. 10.5% of left-handed mothers, χ2[1] = 26.79, p < .001), but no difference was seen for MH (3.7% of mixed-handed fathers vs. 3.6% of mixed-handed mothers, χ2[1] = 0.52, p = .47). In comparison with right-handed, non-right-handed mothers and fathers more often had left-handed twins (non-right-handed mothers 31.2% vs. right-handed mothers 26.9% of left-handed offspring, χ2[1] = 19.45, p < .001; non-right-handed fathers 35.5% vs. right-handed fathers 26.2% of left-handed offspring; χ2 (1) = 80.95, p< .001). No differences were observed for having mixed-handed offspring (non-right-handed mothers 4% vs. right-handed mothers 3.1% of mixed offspring, χ2[1] = 3.45, p = .063; non-right-handed fathers 3.4% vs. right-handed fathers 3.2% of mixed-handed offspring; χ2[1] = 0.14, p = .71). When one parent or both parents were non-right-handed, the risk of having left-handed offspring increased on 4.2% and 10.2% respectively, and the risk of having mixed-handed offspring by only 0.2%. The prevalence of offspring handedness as a function of parental handedness is presented in Supplementary Table 4.
Associations of Handedness With Early Life Characteristics
The odds ratios of left- versus right-handed, mixed- versus right-handed, and non-right- versus right-handed twins for 23 early life characteristics are presented in Figure 1. To correct for relatedness between twins in tests of the significance of the associations between 23 characteristics and handedness, univariate analyses were done with GEE logistic regression (see Table 2 and Supplementary Table 5). A higher probability of being left-handed, mixed-handed or non-right-handed was observed (p < .0011) in boys (LH OR =1.2, 95% CI [1.14, 1.26]; MH OR = 2.27, 95% CI [1.93, 2.67]; NRH OR =1.26, 95% CI [1.2, 1.32]), and children born with shorter gestational age (LH OR = 0.91, 95% CI [0.86, 0.95]; MH OR = 0.78, 95% CI [0.67, 0.92]; NRH OR = 0.90, 95% CI [0.85, 0.94]). In twins who were born preterm, the prevalence of LH (15.58%) and MH (1.5%) was higher than in term-born twins (14.39% left-handed, p < .001, and 1.21% mixed-handed, p = .019) (see Supplementary Table 6).

Figure 1. Odds ratio of early life characteristics in left-handed, mixed-handed, or non-right-handed twins. The plot shows the odds ratio (OR) with the 95% confidence interval (CI). Note: *Indicates the early life characteristics that resulted in significance in the GEE regression model (see Table 2)
Table 2. Results (regression coefficients) from univariate analysis of handedness and early life characteristics in twins with correction for relatedness
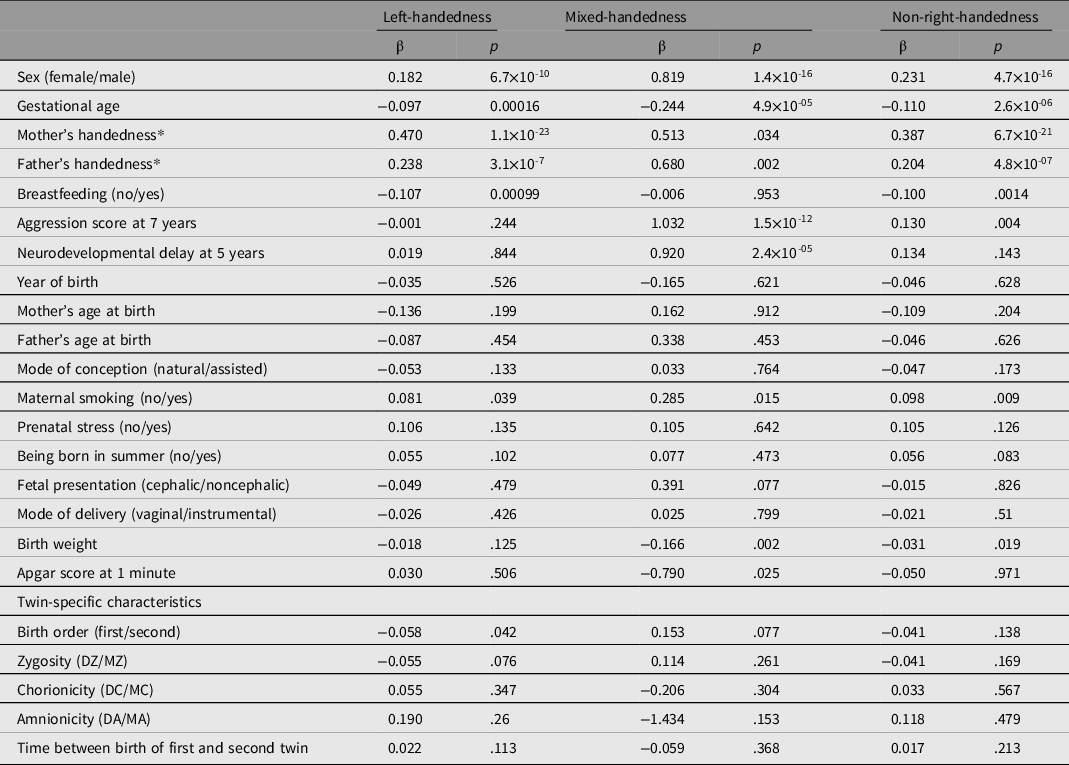
Note: β, regression coefficient; p value in regression with correction for relatedness (GEE); MZ, monozygotic; DZ, dizygotic; MC, monochorionic; DC, dichorionic; MA, monoamniotic; DA, diamniotic. Greg highlighted section, early life characteristics significant for one of three definitions of handedness (α = .001). See Supplementary Table 5 for standard errors and z statistics.
*Mother’s and father’s handedness are included in the regression analysis in the same definition as the twin handedness.
Parental LH increased the risk of being left-handed in offspring (mother: OR = 1.60, 95% CI [1.48, 1.73]; father: OR = 1.27, 95% CI [1.18, 1.37]). Parental NRH also increased the risk of being non-right-handed in offspring (mother: OR = 1.47, 95% CI [1.38, 1.58]; father: OR = 1.23, 95% CI [1.15, 1.31]), whereas the association between parental and offspring’ MH was not significant.
We observed a decrease of LH in twins who were breastfed in childhood (OR =0.90, 95% CI [0.85, 0.95]). Notably, mixed-handed twins had a 2.5-fold higher risk of neurodevelopmental delays at age 5 (OR = 2.51, 95% CI [1.75, 3.59]), and almost twofold higher risk of externalizing problems measured as aggression at age 7 (OR = 1.96, 95% CI [1.53, 2.51]).
Next, the seven significantly associated characteristics (sex, gestational age, mother’s and father’s handedness in the same definition as child’s handedness, breastfeeding, aggression at age 7, and neurodevelopmental delay at 5 years) were included in a multivariate general linear model with each definition of handedness as its own dependent variable. All characteristics together explained 1.25% of the variation in LH, 7.82% in MH, and 1.3% in NRH on the liability scale.
Association of Handedness With Twin-Specific Characteristics
Both LH and MH were equally common in MZ and DZ twins (15.52% of left-handed MZ vs. 14.8% of left-handed DZ twins, χ2[1] = 3.36, p = .067; and 1.44% mixed-handed MZ vs. 1.62% mixed-handed DZ twins, χ2[1] = 1.37, p = .242), and in first- and second-born twins (15.44% left-handed first-born vs. 14.69% second-born, χ2[1] = 3.97, p = .046; and 1.44% mixed-handed first-born vs. 1.68 % second-born χ2[1] = 2.78, p = .095).
We tested the difference in the prevalence of LH and MH in girls from the opposite-sex and same-sex twin pairs and in boys from the opposite-sex and same-sex twin pairs, but did not observe significant differences in LH and MH related to sex of co-twin (left-handed girls from the same-sex and opposite-sex pairs χ2[1] = 5.72, p = .017, and left-handed boys from the same-sex and opposite-sex pairs χ2[1] = 1.3, p = .255; mixed-handed girls χ2[1] = 0.73, p=.393, and mixed-handed boys χ2[1] = 1.7, p = .197; see Supplementary Table 7). None of the twin-specific characteristics were associated with handedness.
Discussion
We investigated the associations between handedness at 5 years of age and a large set of early life characteristics in a population-based sample of twins from the Netherlands Twin Register. Some of the twin-specific characteristics have not been analyzed before, including amnionicity, time interval between the birth of the first and second twin, the neurodevelopmental indices of bowel and bladder toilet skill delays at age 5, and externalizing problems measured as aggression at age 7. Taken together, seven early life characteristics that had independent effects on handedness (namely sex, gestational age, mother’s and father’s handedness in the same definition as child’s handedness, breastfeeding, aggression at age 7, and neurodevelopmental delay at 5 years) in our study explain 1.3% of the variance in NRH, 1.25% in LH, and 7.4% in MH.
Our results show a higher prevalence of LH in twins (14.86%) compared to the adult singleton population (6.97%; Pfeifer et al., Reference Pfeifer, Schmitz, Papadatou-Pastou, Peterburs, Paracchini and Ocklenburg2022)), consistent with the results of a recent meta-analysis of 59 twin studies (Pfeifer et al., Reference Pfeifer, Schmitz, Papadatou-Pastou, Peterburs, Paracchini and Ocklenburg2022) that reported higher prevalence of LH in twins compared to singletons. However, Pfeifer et al. note that the conclusion on the association of twinning with handedness is unsafe because the meta-analysed studies are unadjusted to other characteristics (such as gestational age and birth weight). We note that no evidence for prevalence differences was found in the within-family comparison of twins and their non-twin siblings (Medland et al., Reference Medland, Duffy, Wright, Geffen, Hay, Levy, van-Beijsterveldt, Willemsen, Townsend, White, Hewitt, Mackey, Bailey, Slutske, Nyholt, Treloar, Martin and Boomsma2009) and that our within-family analysis on parental and offspring’s handedness also indicates small differences. Parental LH was strongly predictive of LH in their twin offspring, which was not the case for MH. These results also are consistent with earlier reports (Fagard et al., Reference Fagard, de Agostini, Huet, Granjon and Heude2021; Johnston et al., Reference Johnston, Nicholls, Shah and Shields2013; Zhu et al., Reference Zhu, Obel, Basso, Bech, Henriksen and Olsen2009). Consistent with the meta-analysis of handedness in twins, we observed comparable prevalence of MH in twins, that is, 1.33% versus 2.67% in singletons (Pfeifer et al., Reference Pfeifer, Schmitz, Papadatou-Pastou, Peterburs, Paracchini and Ocklenburg2022).
We confirmed a higher prevalence of LH and MH in males as has been demonstrated previously (Papadatou-Pastou et al., Reference Papadatou-Pastou, Martin, Munafò and Jones2008; Peters et al., Reference Peters, Reimers and Manning2006), and in preterm-born individuals (<37 weeks) compared to twins born after 37 weeks of gestation. This higher prevalence of NRH in preterm born children was also seen in studies of singleton children at age 5−6 years (Gutteling et al., Reference Gutteling, de Weerth and Buitelaar2007; Marlow et al., Reference Marlow, Hennessy, Bracewell and Wolke2007; van Heerwaarde et al., Reference van Heerwaarde, van der Kamp, van der Aa, de Vries, Groenendaal, Jongmans, Eijsermans, Koopman-Esseboom, van Haastert, Benders and Dudink2020). It is thought that preterm birth interrupts the lateralization process (van Heerwaarde et al., Reference van Heerwaarde, van der Kamp, van der Aa, de Vries, Groenendaal, Jongmans, Eijsermans, Koopman-Esseboom, van Haastert, Benders and Dudink2020) and is a cause of brain injury (Domellöf et al., Reference Domellöf, Johansson and Rönnqvist2011).
We found that MH and LH have different correlates. The most notable finding in our study was the relationship between MH and two later life outcomes, neurodevelopmental delay measured by toilet skills problem at 5 years and externalizing problems measured as aggression at age 7. Our study indicates that one phenotypic marker of increased risk for adverse neurodevelopment could be MH. Mixed-handed twins, but not left-handed twins, had a higher risk of toilet skill problems at 5 years, a factor that is relevant to neurodevelopment, and a higher risk of externalizing problems at 7 years. Mental health problems tend to be more severe in non-right-handed adolescents (van der Hoorn et al., Reference van der Hoorn, Oldehinkel, Ormel, Bruggeman, Uiterwaal and Burger2010).
We found that a lack of breastfeeding is associated with LH (de Kovel et al., Reference de Kovel, Carrión-Castillo and Francks2019; Hujoel, Reference Hujoel2019). Several factors could underlie this association. For example, insufficient lactation in mothers who have had multiple births can cause early breastfeeding cessation (Damato et al., Reference Damato, Dowling, Standing and Schuster2005). There may be common genetic factors that lead to insufficient lactation in mothers and LH in offspring. Infant breastfeeding can be adversely affected by cesarean sections (Hobbs et al., Reference Hobbs, Mannion, McDonald, Brockway and Tough2016), and cesarean sections are more prevalent in twin births. Finally, in comparison to breastfeeding, bottle-feeding can decrease myelination and neurodevelopment (Deoni et al., Reference Deoni, Dean, Piryatinsky, O’Muircheartaigh, Waskiewicz, Lehman, Han and Dirks2013; Hujoel, Reference Hujoel2019). The epigenetic factors may explain the link between breastfeeding and handedness, as epigenetic signals were detected for both breastfeeding and handedness in the Netherlands Twin Register. However, no overlap was observed in the differentially methylated positions associated with breastfeeding (Odintsova, Hagenbeek et al., Reference Odintsova, Hagenbeek, Suderman, Caramaschi, van Beijsterveldt, Kallsen, Ehli, Davies, Sukhikh, Fanos, Relton, Bartels, Boomsma and van Dongen2019) and differentially methylated regions associated with LH (Odintsova et al., Reference Odintsova, Suderman, Hagenbeek, Caramaschi, Hottenga, Pool, Consortium, Dolan, Ligthart, van Beijsterveldt, Willemsen, de Geus, Beck, Ehli, Cuellar-Partida, Evans, Medland, Relton, Boomsma and van Dongen2022).
There was no effect on handedness of season of birth, and of the sharing the womb with a male twin of opposite-sex twin. Both effects have been hypothesized based on a potentially higher prenatal testosterone exposure (Nicholls, Reference Nicholls1998; Vuoksimaa et al., Reference Vuoksimaa, Eriksson, Pulkkinen, Rose and Kaprio2010). The effect of the season of birth on hand preferences was reported, but its explanation remained unclear in the literature (de Kovel et al., Reference de Kovel, Carrión-Castillo and Francks2019). The exposure to higher testosterone level during pregnancy on females from opposite-sex twin pairs was also discussed in association with handedness (Vuoksimaa et al., Reference Vuoksimaa, Eriksson, Pulkkinen, Rose and Kaprio2010), but is inconsistent with the results of studies that actually measured androgen concentrations in girls of opposite-sex twin pairs. For example, Kuijper et al. (Reference Kuijper, Twisk, Korsen, Caanen, Kushnir, Rockwood, Meikle, Hompes, Wit and Lambalk2015) did not find higher androgen concentrations in girls from DZ opposite-sex twin pairs.
We found no evidence that twins’ birth weight is associated with handedness. Birth weight in twins is likely to be affected by different factors than in singletons, as intrauterine growth restrictions play an important role in twin pregnancies (Muhlhausler et al., Reference Muhlhausler, Hancock, Bloomfield and Harding2011). We found no association of handedness with any of the other characteristics such as year of birth, parental age at birth, mode of conception, prenatal smoking, stress during pregnancy, fetal presentation, mode of delivery and Apgar scores in the present study.
With respect to twin-specific characteristics, we did not find differences between handedness in MZ and DZ twins, consistent with other studies in twins (Derom et al., Reference Derom, Thiery, Vlietinck, Loos and Derom1996; Medland et al., Reference Medland, Wright, Geffen, Hay, Levy, Martin and Duffy2003; Pfeifer et al., Reference Pfeifer, Schmitz, Papadatou-Pastou, Peterburs, Paracchini and Ocklenburg2022). As reported by others, we found that the handedness in twins was not associated with chorion or amnion type (Derom et al., Reference Derom, Thiery, Vlietinck, Loos and Derom1996; Medland et al., Reference Medland, Wright, Geffen, Hay, Levy, Martin and Duffy2003). In contrast with these previous studies (Medland et al., Reference Medland, Wright, Geffen, Hay, Levy, Martin and Duffy2003), we found no effect of birth order on handedness. We studied association of handedness with the time between the birth of the first and second twin, which could be an indicator of longer perinatal hypoxia for a second-born twin. As previously suggested, hypoxia is a risk factor for adverse outcomes in the second twin (Bakan, Reference Bakan1977). But we found no effect of intertwin delivery time on handedness.
One limitation of the current study may be that the measurement of handedness was based on parental reports from a single question, but we showed that this item captures nearly all information from other handedness items. Another limitation is the definition of the mixed-handed group. We labeled MH based on the parental responses, but could not ascertain the extent to which children may prefer one hand over the other. Thus, we could not define the ambidexterity group, as suggested by other authors (Papadatou-Pastou et al., Reference Papadatou-Pastou, Ntolka, Schmitz, Martin, Munafò, Ocklenburg and Paracchini2020). The mixed-handed group in our analysis could include both mixed-handed and ambidextrous individuals, as in the recent meta-analysis of Pfeifer et al. (Reference Pfeifer, Schmitz, Papadatou-Pastou, Peterburs, Paracchini and Ocklenburg2022).
The missing data problem tends to be a general limitation of many association studies on handedness and early life characteristics, including the present study. For some participants, data on early life characteristics were missing due to the absence of the relevant questions in earlier surveys (e.g., fetal presentation at birth, mode of conception, maternal stress during pregnancy, Apgar scores). The data on chorionicity and amnionicity, obtained from the Dutch national pathology database, are highly reliable. Unfortunately, these data were available only in a subsample of twins after linking to the NTR data.
In conclusion, associations with handedness (based on three definitions) and a wide range of early life characteristics in a national twin cohort depend on the definition of handedness. Sex and gestational age are associated with all definitions of handedness, but there is some variation in associations with other early life characteristics and LH or MH. LH was associated with parental LH and breastfeeding, and MH was associated with a higher risk of neurodevelopmental delays at 5 years of age, and externalizing problems measured as aggression at 7 years of age. Twin-specific characteristics were not related to handedness.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/thg.2023.23
Acknowledgments
We warmly thank all the twins and their families for their lifelong cooperation with the NTR.
Authors’ contributions
Veronika Odintsova: Formal analysis, Software, Methodology, writing — original draft preparation, visualisation. Conor Dolan: Conceptualization, Methodology, Supervision, Writing — Original draft preparation. Catharina E.M. van Beijsterveld: Data curation; writing — review and editing. Lannie Ligthart: Data curation. Gonneke Willemsen: Data curation; Writing — review and editing. Eco J. C. de Geus: Writing — review and editing. Jenny van Dongen: Writing — review and editing. Dorret Boomsma: Conceptualization; supervision; funding acquisition; writing — review and editing.
Funding
The authors would like to acknowledge the Netherlands Organization for Scientific Research (NWO) and The Netherlands Organisation for Health Research and Development (ZonMW) grants: Twin family database for behavior genomics studies (NWO 480-04-004); Twin research focusing on behavior (NWO 400-05-717); Genotype/phenotype database for behavior genetic and genetic epidemiological studies (ZonMw Middelgroot 911-09-032); ‘Why some children thrive’ (OCW Gravity program – NWO-024.001.003); Netherlands Twin Registry Repository: researching the interplay between genome and environment (NWO-Groot 480-15-001/674); Spinozapremie (NWO- 56-464-14192) and KNAW Academy Professor Award (PAH/6635) to DIB; Amsterdam Public Health (APH) and Amsterdam Reproduction & Development (AR&D). We thank professor Chris McManus for his feedback on the manuscript.
Competing interests
none.






