INTRODUCTION
Amyotrophic lateral sclerosis (ALS), the most common adult-onset motor neuron disease, is characterized by degeneration of upper and lower motor neurons in the brain and spinal cord. Median survival is 2-5 years after symptom onset, but survival times can range from a few months to several decades.Reference Andersen 1 Although many of the cellular processes leading to demise of motor neurons are still not completely understood, several genetic mutations and environmental risk factors have been related to the pathogenesis.Reference Chio, Logroscino and Traynor 2 Irrespective of the cause, downstream pathophysiological events that contribute to neurodegeneration in ALS include oxidative stress and glutamate-mediated excitotoxicity, as well as activation of pro-inflammatory mediators. However, the interaction between these events in this complex disease is still an enigma. Oxidative stress that includes protein injury,Reference Shaw, Forrest, Ince, Richardson and Wastell 3 lipid peroxidaion,Reference Shibata, Nagai and Uchida 4 DNA damageReference Fitzmaurice, Shaw and Kleiner 5 and RNA oxidationReference Chang, Kong and Shan 6 has been reported in patients with either familial (FALS) or sporadic (SALS). ALS.Oxidative stress biomarkers in SALS patients have been repeatedly found in urine, Cerebro Spinal Fluid (CSF), blood, tissues, individually or together, such as elevated malondialdehyde (MDA) modified protein, nuclear DNA 8-hydroxy-2’-deoxyguanosine (8-OHdG)Reference Ferrante, Browne and Shinobu 7 and total antioxidant status (TAS). Low levels of erythrocyte glutathione, low activity of erythrocyte glutathione peroxydase (GPX) and superoxyde dismutase 1 (SOD1)Reference Robelin and Gonzalez De Aguilar 8 have also been described, but some discrepancies related to these findings have been reported. Interestingly, mitochondrial damage and oxidative stress play a major role in ALS even in non-SOD1-dependent cases.Reference Carri, Valle, Bozzo and Cozzolino 9 Numerous environmental factors may contribute to oxidative stress and preclude a discussion of the direct alteration of oxidative stress status. For example some studies found increased concentrations of homocysteine in ALS correlated with progression,Reference Zoccolella, Simone and Lamberti 10 alterations of vitamin and metals levels,Reference Lovejoy and Guillemin 11 all mechanisms able to modify oxidative status of patients. Inflammation, another major pathophysiological process involved in ALS, has been reported by fundamental studies of cultured cells, animal models and clinical studies in humans.Reference Bowerman, Vincent, Scamps, Perrin, Camu and Raoul 12 Although oxidative stress and inflammation are non- ALS-specific, their exploration may improve the knowledge of ALS pathogenesis. Thus we conducted a preliminary study to identify some relevant blood markers of oxidative stress and inflammation, and to discuss their link to ALS. Such explorations may help identify circulating biomarkers useful in routine practice. We aim to determine the feasibility of incorporating these techniques into biomarkers research. The findings of this pilot study could also suggest some possible pathophysiological pathways.
METHOD
Patients
We enrolled 10 patients with definite or probable sporadic ALS according to the revised El Escorial criteria.Reference Brooks, Miller, Swash and Munsat 13 All patients were evaluated in the ALS center of Tours, France, in 2014. The site of onset was defined as bulbar or limb. Age at onset was defined as the time of occurrence of first motor weakness noted by the patient. We determined the Revised ALS Functional Rating Scale score (ALSFRS-r score) at diagnosis as well as its change during the first six months after diagnosis and the progression rate (48-ALSFRS) at diagnosis/disease evolution. We also collected data on degree of weight loss at diagnosis (calculated as the weight at diagnosis subtracted from the usual adult weight reported by the patient), considered an excellent nutrition and prognosis marker in ALS. Patients were screened for C9ORF72, SOD1, FUS, and TDP43 gene mutations. All patients were treated with riluzole from the date of blood collection, corresponding to the diagnosis date. We also recruited 10 gender and age-matched controls without any neurological disorders. We collected data about smoking for all subjects. No subject was taking vitamin E supplements.
Blood samples obtained during routine examination of patients at diagnosis were prospectively collected and the remaining volume was used for research. Blood samples were collected with the same procedures in patients and controls. The Ethics Committee of Tours Hospital approved the research on samples collected for routine care. Each participant signed a consent form after reviewing the details of the study.
Markers of oxidative stress
In order to monitor the biological effect of therapeutic aganes we chose different markers of oxidative stress according to 1) the relevance of these biomarkers to ALS, 2) their capacity to accurately identify oxidative stress, 3) the reliability of the measurement method and 4) the opportunity to suggest new therapeutic targets.
TAS was studied in serum as previously described.Reference Erel 14 Glutathione disulfide (GSSG) and reduced glutathione (GSH) levels in whole blood, MDA levels in plasma, and 8-OHdG levels in serum were determined according to published methods.Reference Garçon, Leleu and Marez 15 , Reference Corazao-Rozas, Guerreschi and Jendoubi 16 , Reference Dergham, Lepers and Verdin 17 Antioxidant enzyme activities of SOD, GPX and GR in whole blood were carried out using commercially available kits (Sigma-Aldrich, Saint-Quentin-Fallavier, France). The methemoglobincyanide method was used to determine Hb contents in whole blood.
Other parameters
We measured the concentrations of 5 vitamins: serum and erythrocyte B9, B12 (Immulite 2000®, Siemens Healthcare, France), A, E, C (High Performance Liquid Chromatography, Chromsystems Instruments and Chemicals GmbH, Germany), homocysteine (Liquid chromatography coupled with mass spectrometry) and the following metals: Cu, Zn, Mn, Se (Atomic Emission Spectrometry by High Frequency Plasma) and Fe (C16000®, Abott, France). All these methods are standard and validated for routine practice with rigorous quality programs and are accredited or in the course of accreditation by the National Quality Program (France).
Cytokine concentrations (Tumor Necrosis Factor-alpha, TNFα; Interleukin-8, IL-8; Interleukin-6, IL-6; Interleukin-10, IL-10) in serum were determined using a MILLIPLEX® MAP Human Cytokine/Chemokine Magnetic Bead Panel-Immunology Multiplex Assay (Millipore SAS, Molsheim, France).
Statistical analysis
Biochemical variables were compared between ALS patients and controls using a non-parametric Wilcoxon test. Correlation between levels of oxidative stress markers and quantitative clinical and biological variables was analyzed by Spearman’s rho correlation.
The significance threshold was set at p < 0.05. We performed a pilot study with numerous tests on a small population; therefore, in order to clearly reveal the trends, we did not apply a restrictive correction for multiple test. This correction will be used in a second step when we will confirm the findings on a larger cohort. Statistical analyses were performed with JMP® 7.0 software (SAS Institute®, Cary, U.S.A.).
RESULTS
Patient characteristics
We used the data from 9 ALS patients and 10 controls. We excluded one of the 10 recruited patients because the evolution of the disease did not confirm the ALS diagnosis. The characteristics of subjects are shown in Table 1. The median delay between the first symptoms and the diagnosis was 17.8 months. Mean ALSFRS-r score at diagnosis ranged from 35 to 46 and weight loss at diagnosis from 0 to 7.4%. The median ALSFRS-r decline was 3.8% and ranged from 0 to 20.9%. The percentage of smokers was similar in ALS and controls (about 20%). No patient had mutation in C9ORF72, SOD1, FUS and TDP43 genes.
Table 1 Characteristics of ALS patients and controls

Elevated oxidative stress in ALS patients
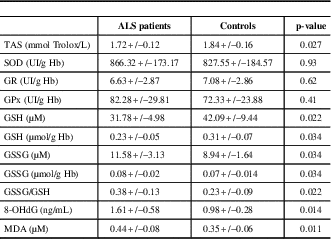
The comparison of oxidative stress markers between ALS patients and controls is shown in Table 1. There was a significant decrease in TAS levels in ALS patients compared to controls (p=0.027). Despite no significant change in antioxidant enzyme activities, the glutathione status was altered in ALS patients, with elevated concentrations of GSSG (p=0.034) and lower concentrations of GSH (p=0.034), leading to a significantly higher GSSG/GSH ratio (p=0.022). There were also deleterious effects of oxidative stress in ALS patients, including elevated concentrations of 8-OHdG and MDA (p=0.014 and 0.011, respectively, Table 2).
Table 2 Comparison of oxidative stress markers between ALS patients and controls
Elevated inflammation in ALS patients
The comparison of inflammatory markers between ALS patients and controls is shown in Table 3. We did not detect any sign of systemic inflammation (normal values CRP and leucocytes--not shown). We observed significantly higher concentrations of IL-6 (p=0.0079) and IL-8 (p=0.009) and a trend to higher concentrations of TNFα in ALS patients (p=0.06).
Table 3 Comparison of inflammatory markers between ALS patients and controls

Other related factors
The measurement of all other parameters--metals, vitamins and metabolic markers--are summed up in Table 4. We did not observe any difference in homocysteine, vitamin and metals concentrations between ALS patients and controls.
Table 4 Exploration of other factors : metal, vitamin, and homocysteine measurement

Relation between biological and clinical parameters
We found an inverse relation between homocysteine concentrations and ALSFRS-r at diagnosis (R2=0.55, p=0.02) (Figure 1A) and a positive correlation between Cu levels and ALFRS-r decline (R2=0.84, p=0.0037) (Figure 1B).

Figure 1 Correlation between biological and clinical parameters A: ALSFRS-r at diagnostic vs homocysteine levels (p=0.02) , B : ALSFRS-r decline vs Cu levels (p=0.0037).
Relation between markers of oxidative stress and inflammation
We found a positive correlation between IL-6 concentrations and the ratio GSSG/GSH (R2 = 0.46, p=0.045) and an inverse correlation between IL-6 concentrations and SOD activity (R2 = 0.58, p=0.017) (Figures 2A and 2B). Importantly, we did not observe any significant relation between these parameters in controls. There is substantial evidence implicating oxidative stress as a central mechanism in ALS patients, the ultimate trigger that causes increased ROS levels is still largely unknown, leading to speculation as to whether oxidative stress is a primary cause of the disease or merely a consequence of some other toxic insult. Moreover, it is still unclear whether oxidative stress is involved outside of the CNS; reliable oxidative stress biomarkers, especially blood biomarkers, are the first essential step to ascertaining such extra-CNS involvement. A better understanding of oxidative stress may also help to suggest some therapeutic targets and to act upstream of cellular damage.

Figure 2 Correlation between oxidative stress and inflammatory markers : A GSSG/GSH vs IL-6 levels (p=0.045), B: SOD activity vs IL-6 levels (p=0.017).
This pilot study suggests that oxidative stress and inflammatory parameters are relevant factors in the evaluation of ALS. Although performed on a small sample, the strengths of this study are the exploration of multiple parameters outside of the CNS such as oxidative stress and inflammatory factors and the concomitant evaluation of other factors (e.g. enzyme cofactors, metabolic parameters) that is rarely performed.
We found a significant decrease of TAS levels in ALS patients, suggesting a serious imbalance between the production of ROS and the ability of the system to remove or repair the damage. Most studies, including ours, are unanimous about the types of biological consequences of oxidative stress. We noted elevated concentration of 8-OHdG that characterizes the oxidative injury to DNA and higher concentrations of MDA considered as a product of lipid peroxydation. A compelling study published by Bogdanov et al.,Reference Bogdanov, Brown and Matson 18 notably found that plasma levels of 8-OHdG in ALS were significantly higher than in the group with no neurological disorders but not in the group with other neurological disorders. Moreover, Mitsumoto et al.Reference Mitsumoto, Santella and Liu 19 revealed increased urinary 8-OHdG. Oxidative stress reactions can also significantly alter membrane structure and intracellular lipids, resulting in altered fluidity, permeability, transport and metabolic processes.Reference Parakh, Spencer, Halloran, Soo and Atkin 20 Fatty acid peroxides can give rise to a variety of aldehydes, such as 4-hydroxy-2-nonenal (HNE)Reference Parakh, Spencer, Halloran, Soo and Atkin 20 and thiobarbituric acid-reactive substances (TBARS) like MDA, that are not only end products and remnants of lipid peroxidation processes but also “second cytotoxic messengers.” Moreover GSH, a free-radical scavenger tripeptide that regulates the intracellular redox state, was reported lower in ALS, associated with an increase of its oxidized form that could be deleterious for the disease evolution.Reference Weiduschat, Mao and Hupf 21 , Reference Babu, Kumar, Chandra, Puri, Kalita and Misra 22 , Reference Babu, Kumar and Chandra 23 We confirmed these findings with a significantly increased GSSG/GSH ratio in ALS patients. Contrary to other reports we did not observe any alteration of GR, GPX and SOD1 activity in ALS patients.Reference Babu, Kumar, Chandra, Puri, Kalita and Misra 22 However, the role played by SOD1 in ALS is not clear in the literature. According to one report SOD1 is not particularly protected against its inactivation by ROS, a process that could contribute to its partial inactivation.Reference Garcon, Shirali and Garry 24 The genetic status of SOD1 or the state of the disease at the time of exploration may explain the discrepancies between studies. We also have to take into account that numerous studies about oxidative stress are preliminary, with a small number of patients, different methodologies and often without exploring the enzyme cofactors.
Moreover, we reported no statistically significant change in the levels of the vitamins A, C, and E in the sera of ALS patients as compared to controls. These three vitamins are generally considered to be some of the more biologically active non-enzymatic antioxidant.Reference Garcon, Garry and Gosset 25 Vitamin E might have a protective role in preventing the development of ALS while also slowing disease progression, but this effect is controversial.Reference D’Amico and Bertini 26
Interestingly, we did not find any significant increase in homocysteine and metals concentrations as observed in some other studies.Reference Ingre, Roos, Piehl, Kamel and Fang 27 According to other authors, however, exposure to metalsReference Callaghan, Feldman, Gruis and Feldman 28 is a suspect in the search for the cause of ALS,Reference Lovejoy and Guillemin 11 with many case reports linking different metals to ALS. In ALS there may be a direct link between iron, oxidative stress and regional neurodegeneration.Reference Garcon, Shirali and Garry 24 The findings about oxidative stress were reinforced by the absence of the influence of enzyme cofactors or other sources of oxidative stress. This study is a proof of concept that the exploration of all these markers of oxidative stress could be feasible and independently from some other factors linked to this way.
Neuroinflammation in now established as an important factor in the pathogenesis of ALS We observed higher concentrations of IL-6, and IL-8 that have been previously describedReference Bowerman, Salsac and Coque 29 and that confirmed the pro- inflammatory and/or hypoxemia state of ALS patients at the first stage of the disease. The role of IL-6 in several neurological diseases and its link with iron metabolism led some authors to target these ways, such as the blockade of IL-6 signalisation.Reference Fiala, Mizwicki, Weitzman, Magpantay and Nishimoto 30 Recent research showed that this neuroinflammatory component is affected by the peripheral immune system; T lymphocytes in particular are able to cross into the brain and spinal cord parenchyma, where they interact with resident microglia, either inducing them to adopt an M1(cytotoxic) or M2 (protective) phenotype, depending on the stage of disease. Interestingly, TNF-α could trigger the induction of oxidative stress in a mechanism involving the NF-κB signalling pathway which could be deregulated in ALS.Reference Prell, Lautenschlager, Weidemann, Ruhmer, Witte and Grosskreutz 31 These data link two important pathogenic mechanisms, oxidative stress and neuroinflammation, suggested to play a role in ALS.Reference Tolosa, Caraballo-Miralles, Olmos and Llado 32 Hence, taken together, our data confirmed oxidative stress as well as inflammation status in the system outside of central nervous system as advocated by Mistumoto et al.Reference Mitsumoto, Santella and Liu 19
Oxidative stress and inflammation are non-ALS-specific but the connection between these and disease progression such as weight loss and decline of ALSFRS-r score may be fruitful to investigate in ALS.Reference Mitsumoto, Factor-Litvak and Andrews 33 For example the association between homocysteine and diagnosis ALSFRS as well as between copper level and ALSFRS-r decline are relevant and consistent with previous findings.Reference Weiduschat, Mao and Hupf 21 , Reference Hozumi, Hasegawa and Honda 34 , Reference Zoccolella, Bendotti, Beghi and Logroscino 35 , Reference Roos, Vesterberg, Syversen, Flaten and Nordberg 36 The limited number of enrolled ALS patients precluded an analysis of the relation between biological markers and long-term disease evolution.
CONCLUSION AND FUTURE PERSPECTIVE
Although non-specific, we observed elevated of oxidative stress and inflammation and a link between some clinical parameters in ALS. Further investigations could help us better understand the link between these mechanisms and iron metabolism as well as the mitochondrial functions, with the aim of identifying the primary and secondary phenomena. All these parameters are probably associated with several factors that contribute to motor neurodegeneration but they have to be included in prognosis studies. These promising results encourage us to follow this study with collection of combined inflammatory and oxidative stress markers, confusion factors and clinical data to rigorously evaluate these relationships. This pilot study is promising but its small size limits the scope of our findings, which need to be further validated. After validation on an independent and larger cohort, the biomarkers identified in this study could also be useful in clinical trials to define some subgroups of patients based on inflammatory and redox status. The strategy of using combined biological markers has to be tested with other types of biomarkers (e.g. metabolic) to more accurately define some biological phenotypes. The most promising opportunity offered by this kind of study would be the identification of new therapeutic targets. Note that numerous therapeutic antibodies have been designed and may target some relevant inflammatory biomarkers identified in ALS and also in other neurological diseases such as Alzheimer’s disease or Multiple Sclerosis.
Disclosures
Hélène Blasco, Guillaume Garcon, Franck Patin, Charlotte Veyrat-Durebex, Charlotte Veyrat-Durebex, Judith Boyer, David Devos, Patrick Vourc’h, Christian Andres, and Philippe Corcia hereby state that they do not have anything to disclose.
Statement of Authorship
Hélène Blasco performed the statistical analysis, interpreted the data, and wrote the first draft of the manuscript. Guillaume Garcon acquired data and critically revised the manuscript for important intellectual content. Charlotte Veyrat-Durebex acquired data. Franck Patin acquired data. Judith Boyer acquired data. David Devos critically revised the manuscript for important intellectual content. Christian R Andres critically revised the manuscript for important intellectual content. Philippe Corcia recruited patients, interpreted data and critically revised the manuscript for important intellectual content.








