Introduction
A strong geographical correlation between infant mortality (from 1921 to 1925) and adult ischaemic heart disease (IHD) mortality (from 1968 to 1978) was observed by Barker and Osmond.Reference Barker and Osmond 1 They postulated that factors that increased the risk of death during infancy also increased susceptibility to IHD among those who survived infancy, and later, showed that blood pressure (BP) in adulthood was positively related to placenta weight but inversely associated with birth weight.Reference Barker, Bull, Osmond and Simmonds 2 They suggested that poor fetal nutrition indicated by intrauterine growth restriction and low birth weight was associated with this increased susceptibility to IHD.
Subsequently, several studies [mainly from high-income countries (HICs)] have investigated the relationship between birth size parameters (e.g. birth weight, head circumference, placenta size) and later cardiovascular disease risk (mainly BP), with birth weight the most widely studied parameter. Results from several of these studies have shown an inverse association between birth weight and BP later in life.Reference Schack-Nielsen, Holst and Sorensen 3 – Reference Pharoah, Stevenson and West 7 A smaller number of studies have reported positive or no association between birth weight and later BP. For example, positive associations have been reported among UK neonatesReference O’Sullivan, Kearney and Crowley 8 and Chinese children,Reference Perng, Rifas-Shiman and Kramer 9 whereas birth weight was not associated with BP among American adolescents.Reference Falkner, Hulman and Kushner 10 The relationship between birth weight and later BP differed by gender among UK adolescents: a negative association was seen in the males but a positive association in the females.Reference Macintyre, Watt, West and Ecob 11 Systematic reviews have reported that, on average, systolic blood pressure (SBP) drops by 2–4 mmHg for every kilogram increase in birth weight.Reference Law and Shiell 12 , Reference Huxley, Shiell and Law 13 These reviews have predominantly comprised of studies among adults from HICs.
In HICs the prevalence of low birth weight varies between 5 and 8%.Reference Kim and Saada 14 Low birth weight is more common in Africa (7% in Nigeria,Reference Dahlui, Azahar, Oche and Aziz 15 8% Uganda,Reference Ndibazza, Muhangi and Akishule 16 11% Zambia,Reference Chibwesha, Zanolini and Smid 17 17% Zimbabwe and BeninReference Assefa, Berhane and Worku 18 , Reference Denoeud, Fievet and Aubouy 19 and 28% EthiopiaReference Assefa, Berhane and Worku 18 ) and on average African population have lower birth weights when compared with European populations.Reference Shiono, Klebanoff, Graubard, Berendes and Rhoads 20 In HICs, low birth weight is predominantly due to prematurity (most commonly as a result of maternal smoking in pregnancyReference Delnord, Blondel and Zeitlin 21 ), whereas, in developing countries, low birth weight for gestational age constitutes most of the low birth weight infants.Reference Garner, Kramer and Chalmers 22 The causes of low birth weight differ between rural, tropical Africa and developed or non-tropical settings: for example, malaria (an important cause of low birth weight) is restricted to the tropicsReference Shulman and Dorman 23 and prophylactic antimalarial drugs in pregnancy reduce the risk of low birth weight.Reference Muanda, Chaabane and Boukhris 24 , Reference Cot, Le Hesran and Miailhes 25 We hypothesized that the relationship between birth weight and BP in African settings might differ from that commonly observed in HICs.
The role of birth weight in the later development of BP is important to African countries: these have a high burden of malnutrition,Reference Akombi, Agho, Merom, Renzaho and Hall 26 – Reference Benzekri, Sambou and Diaw 28 low birth weightReference Assefa, Berhane and Worku 18 , Reference Denoeud, Fievet and Aubouy 19 and raised BP.Reference Guwatudde, Mutungi and Wesonga 29 – Reference Reddy and Mbewu 32 Early life interventions that reduce maternal malnutrition and extremes of birth weight (both low and high) may thus control childhood BP (before clinical manifestation of disease) and could be vital in the prevention of high BP in adulthood.
The absence of birth weight records for adults in many African countries and the low accuracy of maternally recalled birth weightReference Lule, Webb and Ndibazza 33 limits prospects for studying the relationship between birth weight and BP in adulthood in this setting. However, the emergence of a number of birth cohorts (with birth records) in Africa provides opportunities to investigate the relationship between birth weight and BP among African children and adolescents. Childhood BP predicts BP in early adulthood,Reference Kagura, Adair, Musa, Pettifor and Norris 34 , Reference Hao, Wang and Treiber 35 thus studies of the relationship between birth weight and BP among children are important in the identification of at-risk groups for targeted interventions early in life. We conducted a qualitative assessment of the direction and consistency of the relationship between birth weight and BP among African children and adolescents using a systematic review of existing literature.
Methods
A literature search covering publications up to 15 October 2016 with no restriction on start date was performed using Medline, EMBASE, Global Health and Web of Science databases. The search was performed on combinations of the keywords: (hypertension OR blood pressure) AND (birth weight) AND (paediatric OR child OR young people OR youth OR juvenile OR adolescent OR youngster OR pubescent OR teenage OR new-born OR minor OR infant) AND (Africa OR individual names of countries in Africa).
Original papers on the relationship between birth weight and BP among children and or adolescents, between ages 0 and 19 years and resident in Africa were reviewed. No restrictions on language or publication dates were applied. Publications on children and or adolescents of African ancestry not residing in Africa were excluded. Papers on the same participants were considered as one study. If more than one paper reported on the same participants at the same age, the most complete paper was included in the review. Papers reporting on the same participants were included and reviewed separately if they reported on the relationship between birth weight and BP at different ages. No additional information was sought from authors. Reference lists of the included papers were searched for additional relevant publications.
Search results were exported to Endnote reference management software (Thomson Reuters, version ×7) and duplicates removed. Two independent authors (S.L. and E.W.) assessed titles and abstracts for inclusion in the full-text review and then assessed full-text articles for inclusion in the data synthesis. Inconsistencies were discussed and consensus reached at each stage of the selection process.
Data were extracted independently by two authors (S.L. and E.W.) using standardized data extraction sheets on the year of publication, year of birth, location, age of participants, study design, number of participants, exclusion criteria, study aim, mean birth weight, source of birth weight data, BP measurement procedure, mean BP [SBP and, or, diastolic blood pressure (DBP)], relationship between birth weight and BP and how this was assessed, and whether there was adjustment for confounders. Information was recorded as presented in the original publication, except where the overall mean BP or birth weight was missing; in this case, where possible the overall mean was calculated from any stratum-specific means presented. Studies were assessed for selection bias and adjustment for confounding. Meta-analysis was not performed due to diversity in studies included in the review, in terms of their design, analysis, source population and covariates controlled for in the analysis. Guidelines from the preferred reporting items for systematic review and meta-analysis (PRISMA)Reference Moher, Shamseer and Clarke 36 were followed.
Results
A total of 990 published abstracts were retrieved from four databases, of these 366 duplicates were removed, leaving 624 abstracts for review (Fig. 1). Of these, 562 were excluded and of the remaining 62 papers that were subjected to full-text review, 46 were excluded. Of the 46 papers excluded, two papersReference Steyn, de Wet and Richter 37 , Reference Sadoh and Ibhanesebhor 38 were duplicates (reported on the same participants at the same age) of one of the included papers. Thus, 16 papers from 13 studies describing, but not necessarily focussing on, the relationship between birth weight and BP were included in the final review and data extraction (Fig. 1).
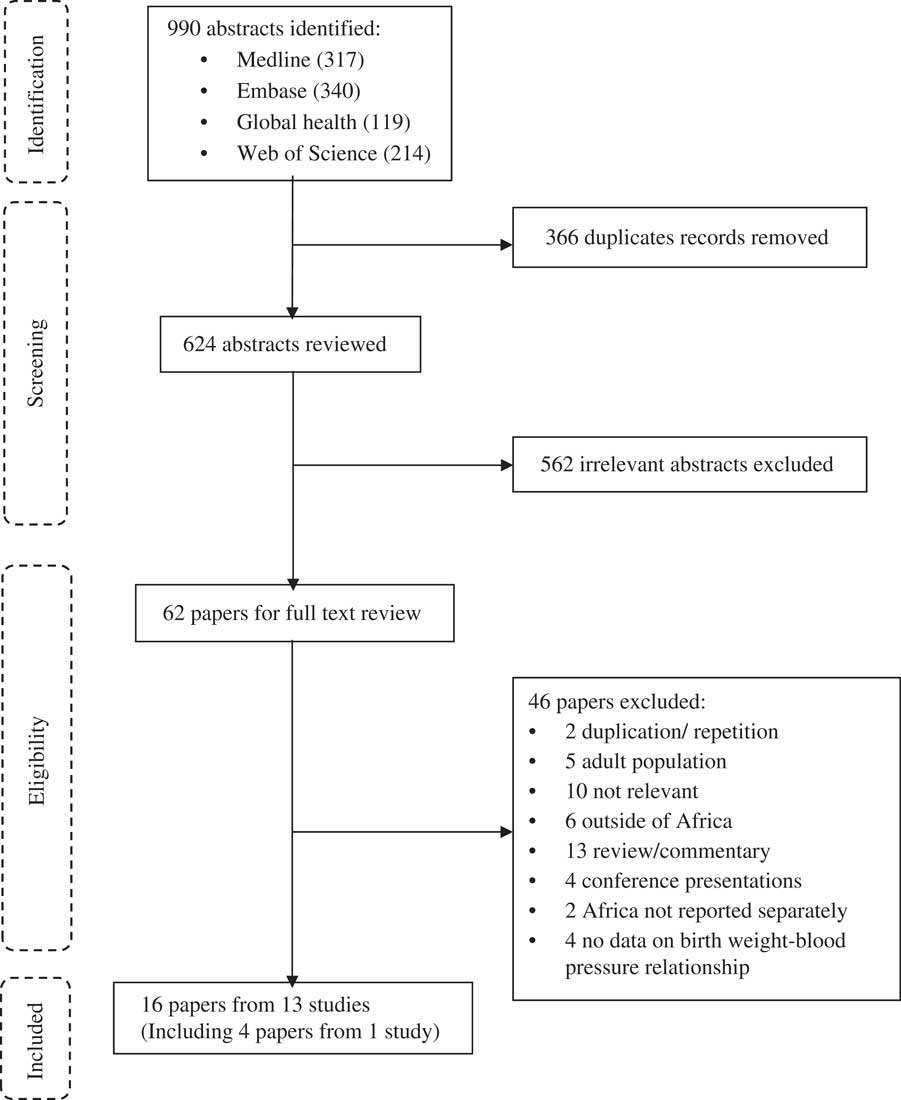
Fig. 1 Flow diagram for systematic review.
Of the 16 papers reviewed, six were from West Africa,Reference Law, Egger and Dada 39 – Reference Nwokoye, Uleanya and Ibeziako 44 six Southern Africa,Reference Silva, Capingana and Magalhaes 45 – Reference Woelk, Emanuel, Weiss and Psaty 50 two Central Africa,Reference Youmbissi, Oudou, Mbede and Nasah 51 , Reference Longo-Mbenza, Ngiyulu and Bayekula 52 one East AfricaReference Chiolero, Paradis and Madeleine 53 and one North Africa.Reference Salvi, Meriem and Temmar 54 Four papers from Southern Africa were from the same cohort but presented data on BP at different ages of follow-up.Reference Kagura, Adair, Munthali, Pettifor and Norris 46 – Reference Levitt, Steyn and De Wet 49 Four papers reported results in neonates (0–28 days),Reference Ayoola, Gemmell and Omotade 41 , Reference Sadoh, Ibhanesehbor, Monguno and Gubler 43 , Reference Nwokoye, Uleanya and Ibeziako 44 , Reference Youmbissi, Oudou, Mbede and Nasah 51 four in children (1–9 years),Reference Law, Egger and Dada 39 , Reference Margetts, Rowland and Foord 40 , Reference Levitt, Steyn and De Wet 49 , Reference Woelk 55 four in adolescents (10–19 years)Reference Hawkesworth, Prentice, Fulford and Moore 42 , Reference Adair, Fall and Osmond 47 , Reference Griffiths, Sheppard and Johnson 48 , Reference Salvi, Meriem and Temmar 54 and four in both children and adolescents.Reference Silva, Capingana and Magalhaes 45 , Reference Kagura, Adair, Munthali, Pettifor and Norris 46 , Reference Longo-Mbenza, Ngiyulu and Bayekula 52 , Reference Chiolero, Paradis and Madeleine 53 The papers were published between 1989 and 2016.
The main characteristics of the reviewed papers are shown in Table 1. Briefly, all papers included both males and females. The number of participants ranged from 157 to 2743 individuals per paper, with five papers reporting on more than 1000 participants. Seven papers had less than 500 participants. Two of the reviewed papers did not present quantitative information on the relationship between birth weight and BP. Eleven of the papers (from eight studies) described results from cohorts, while five papers reported results from cross-sectional studies.
Table 1 Description of the studies included in the systematic review
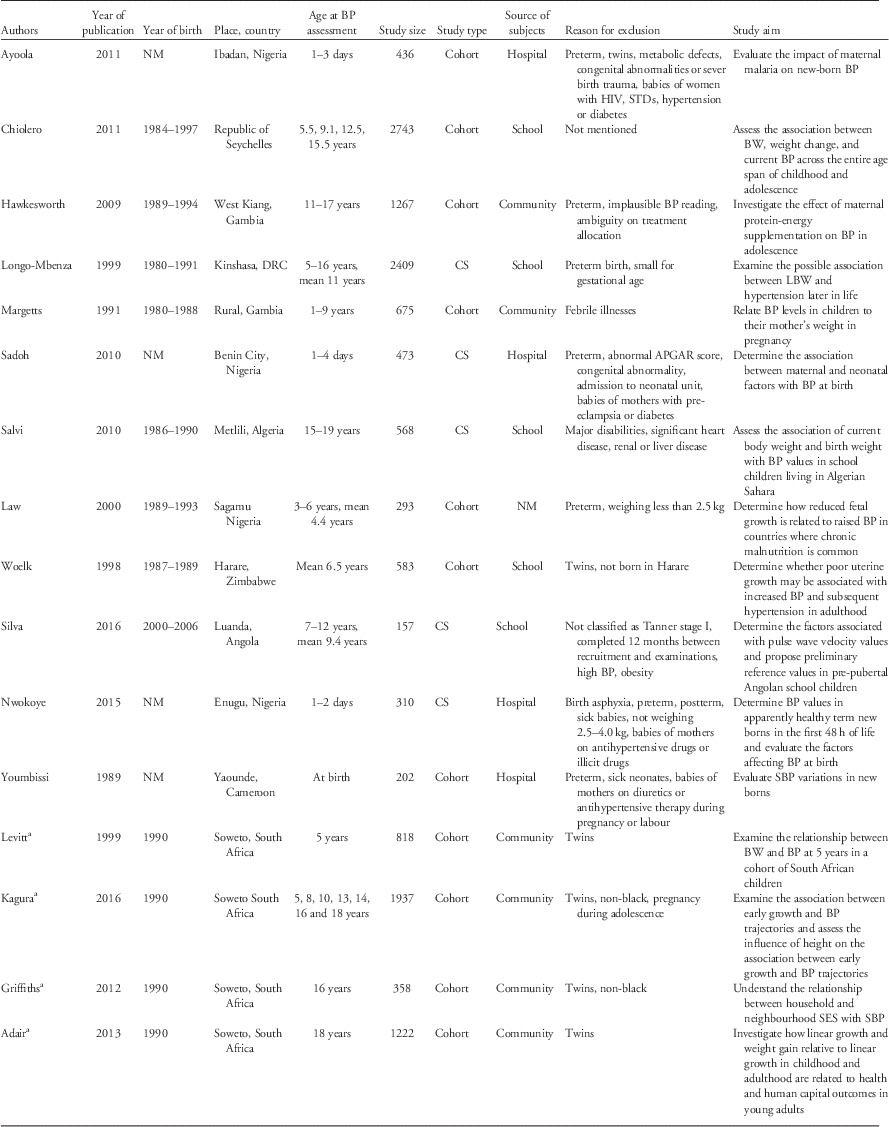
BP, blood pressure; BW, birth weight; DRC, Democratic Republic of Congo; CS, cross-sectional study; LBW, low birth weight; NM, not mentioned; SBP, systolic blood pressure; SES, socioeconomic status.
a Papers from the same cohort (Birth to Twenty cohort).
Except for one paper, in which the source of participants was unclear, participants were recruited from schools (n=5),Reference Silva, Capingana and Magalhaes 45 , Reference Woelk, Emanuel, Weiss and Psaty 50 , Reference Longo-Mbenza, Ngiyulu and Bayekula 52 – Reference Salvi, Meriem and Temmar 54 hospitals (n=4)Reference Ayoola, Gemmell and Omotade 41 , Reference Sadoh, Ibhanesehbor, Monguno and Gubler 43 , Reference Nwokoye, Uleanya and Ibeziako 44 , Reference Youmbissi, Oudou, Mbede and Nasah 51 and communities (n=6; representing three studies).Reference Margetts, Rowland and Foord 40 , Reference Hawkesworth, Prentice, Fulford and Moore 42 , Reference Kagura, Adair, Munthali, Pettifor and Norris 46 – Reference Levitt, Steyn and De Wet 49 Preterm children were excluded in seven papers: all four of the hospital-based studies,Reference Ayoola, Gemmell and Omotade 41 , Reference Sadoh, Ibhanesehbor, Monguno and Gubler 43 , Reference Nwokoye, Uleanya and Ibeziako 44 , Reference Youmbissi, Oudou, Mbede and Nasah 51 one school-basedReference Longo-Mbenza, Ngiyulu and Bayekula 52 and one community-based.Reference Hawkesworth, Prentice, Fulford and Moore 42 Twins were excluded in six papers (representing three studies).Reference Ayoola, Gemmell and Omotade 41 , Reference Kagura, Adair, Munthali, Pettifor and Norris 46 – Reference Griffiths, Sheppard and Johnson 48 , Reference Woelk, Emanuel, Weiss and Psaty 50 Children who were small for gestational age or who weighed <2.5 kg at birth were excluded from three papers; one in neonates,Reference Nwokoye, Uleanya and Ibeziako 44 one in childrenReference Law, Egger and Dada 39 and one in children and adolescents.Reference Longo-Mbenza, Ngiyulu and Bayekula 52 There was variability in the study aims of the papers included: only six described assessing the association between birth weight and BP as one of their main aims.Reference Law, Egger and Dada 39 , Reference Woelk, Emanuel, Weiss and Psaty 50 , Reference Longo-Mbenza, Ngiyulu and Bayekula 52 – Reference Salvi, Meriem and Temmar 54 , Reference Levitt, Lambert and Woods 56
Table 2 summarizes birth weight and BP measurements and values, statistical analysis methods and the relationship between birth weight and BP in the reviewed papers.
Table 2 Main results from the studies included in the systematic review
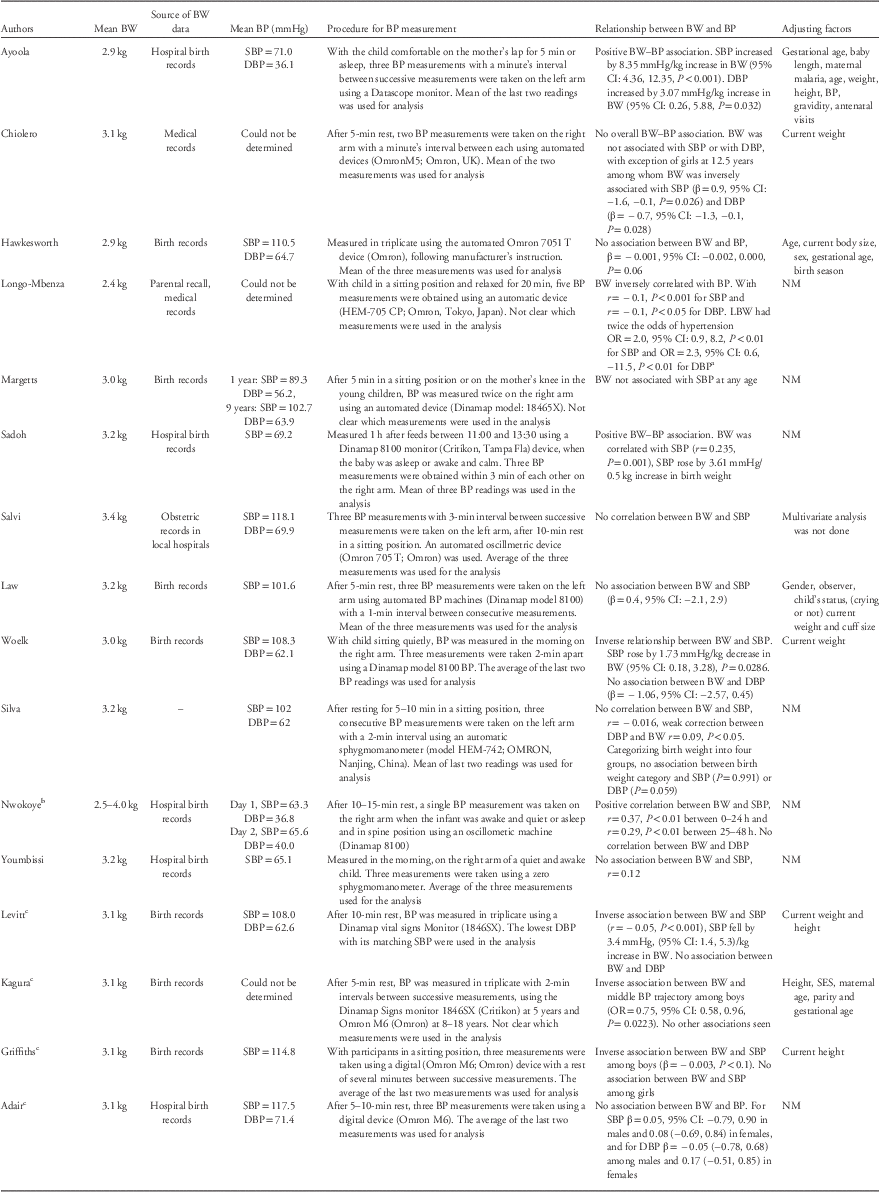
BW, birth weight; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; CI, confidence interval; LBW, low birth weight; OR, odds ratio; NM, not mentioned; SES, socioeconomic status; r, correlation coefficient; β, linear regression coefficient.
a 95% CI and P-value reported as in paper but are inconsistent.
b Mean birth weight not reported and could not be calculated (determined).
c Papers from the same study group (Birth to Twenty cohort).
Birth weight ascertainment
Birth weight was either measured and recorded immediately after birth (predominantly in the cohort studies), or extracted from birth or child health card records. In one study,Reference Longo-Mbenza, Ngiyulu and Bayekula 52 parentally recalled birth weight was used when birth records were missing (in an unknown number of participants). Mean birth weight varied from 2.4 to 3.4 kg.
BP assessment
BP procedures were relatively similar across studies. All studies used automated devices, except for one study, which used the sphygmomanometer machine. Eleven papers reported a resting period (from 5 to 20 min) before proceeding with measuring the BP. In four studies, measurements were taken on the left arm, whereas in six studies measurements were on the right arm; the remaining studies did not include this information.
BP was measured in triplicates in the majority of papers (n=12) with one paper reporting single measurement, two reporting double measurement and one reporting five measurements. The rest period between consecutive BP measurements varied from 1 to 3 min.
Of the 12 papers that measured BP in triplicate, five used the mean of all three measurements in data analysis, five used the mean of the last two measurements, one used the lowest DBP (with matching SBP) and one was unclear. For the paper where BP was measured five times and one of the two papers where BP was measured in duplicate, it was unclear which measurements or combination thereof were used for data analysis. In the other paper where BP was measured in duplicate, the mean of the two measurements was used in the analysis.
Among neonates, mean SBP varied from 65.1 to 71.0 mmHg and in children and adolescents from 89.3 to 118.1 mmHg. Mean DBP varied from 36.1 to 63.9 mmHg in neonates and between 56.2 and 71.4 mmHg among children and adolescents. Mean SBP and DBP generally increased with age over the course of childhood and adolescence, this was especially apparent in the four papers that reported results from the same cohort study at different ages.
Birth weight and BP relationship
The relationship between birth weight and SBP varied across papers; seven papers reported no association,Reference Law, Egger and Dada 39 , Reference Margetts, Rowland and Foord 40 , Reference Hawkesworth, Prentice, Fulford and Moore 42 , Reference Silva, Capingana and Magalhaes 45 , Reference Adair, Fall and Osmond 47 , Reference Youmbissi, Oudou, Mbede and Nasah 51 , Reference Salvi, Meriem and Temmar 54 six an inverse associationReference Kagura, Adair, Munthali, Pettifor and Norris 46 , Reference Griffiths, Sheppard and Johnson 48 – Reference Woelk, Emanuel, Weiss and Psaty 50 , Reference Longo-Mbenza, Ngiyulu and Bayekula 52 , Reference Chiolero, Paradis and Madeleine 53 and three a positive association.Reference Ayoola, Gemmell and Omotade 41 , Reference Sadoh, Ibhanesehbor, Monguno and Gubler 43 , Reference Nwokoye, Uleanya and Ibeziako 44 Among the neonates, three out of four papers reported a positive association while one reported no association. Of the four papers in children, two reported inverse associations and two no association. The papers on children and adolescents predominately found inverse associations (three out of four papers) while among adolescents, three papers found no association and one an inverse association.
Of the seven papers with participant size less than 500 individuals,Reference Law, Egger and Dada 39 , Reference Ayoola, Gemmell and Omotade 41 , Reference Sadoh, Ibhanesehbor, Monguno and Gubler 43 – Reference Silva, Capingana and Magalhaes 45 , Reference Griffiths, Sheppard and Johnson 48 , Reference Youmbissi, Oudou, Mbede and Nasah 51 three papers reported no association between birth weight and SBP, one an inverse association and three a positive association.Reference Sadoh, Ibhanesehbor, Monguno and Gubler 43 – Reference Silva, Capingana and Magalhaes 45 , Reference Youmbissi, Oudou, Mbede and Nasah 51 Studies with larger participant sizes (greater than 500 individuals) were more likely to report inverse associations. Of the nine studies with participant size over 500, three reported no association between birth weight and SBP, five an inverse association and one reported a positive association.
In three of the six papers reporting inverse associations,Reference Kagura, Adair, Munthali, Pettifor and Norris 46 , Reference Griffiths, Sheppard and Johnson 48 , Reference Chiolero, Paradis and Madeleine 53 analyses were conducted at different ages and, or, separately for males and females, with inverse associations only seen among particular subgroups (girls at 12.5 years,Reference Chiolero, Paradis and Madeleine 53 boys onlyReference Kagura, Adair, Munthali, Pettifor and Norris 46 , Reference Griffiths, Sheppard and Johnson 48 ) and analyses from other subgroups showing no evidence of association.
Analysis approaches used to assess the relationship between birth weight and BP were diverse, varying from simple correlation analysis with no adjustment for potential confounders, to more complex group-based trajectory modelling approaches. Multivariable analysis, adjusting for potential confounder(s) [often including age, sex or body size (weight or height)] was conducted in eight papers, of these five reported an inverse association, two no association and one a positive association. In comparison, of the eight papers that did not undertake adjustment for confounders, one reported an inverse association between birth weight and SBP, five reported no association and two a positive association.
The relationship between birth weight and DBP was described in eight papers; a positive association was seen in two papers, inverse association in two papers and no association was reported in four papers. Of these eight papers, two were in neonates, two in children, three in children and adolescents, and one in adolescents only.
Discussion
Overall, this systematic review of existing literature showed varied results. We identified 16 papers from 13 studies addressing the question of whether the inverse relationship between birth weight and BP in later life seen in Western settings is also present in Africa. The relatively small number of studies and their heterogeneity in design and analysis prohibits definitive conclusions. However, we found some evidence to suggest that the relationship between birth weight and SBP in Africa varies with the participants’ age: positive associations were seen in neonates and inverse associations mainly in children. Among adolescents, the relationship was either inverse or showed no evidence of association. Only a few papers reported on the relationship between birth weight and DBP, with most papers reporting no relationship between birth weight and DBP.
This review supports an earlier review by LawReference Law and Shiell 12 that did not include any of the papers reviewed herein (only two of the papers included in the present review had been published at the time of the Law review, and of these Margetts et al.Reference Margetts, Rowland and Foord 40 was excluded for missing quantitative information while Youmbissi et al.Reference Youmbissi, Oudou, Mbede and Nasah 51 was not mentioned). The Law review reported inconsistencies in the relationship between birth weight and BP, especially among adolescents. Generally, inverse associations were among children and positive associations in neonates, inconsistencies could be due to differences in age, sample sizes and statistical analysis approaches. Interestingly, results from the cohort studies included in our review, that measured BP at more than one-time point (different ages), did not show evidence of increasing strength of the association between birth weight and BP with age as reported by the earlier review.Reference Law and Shiell 12 Studies in neonates consisted mainly of less than 500 participants and reported positive associations. Studies with smaller participant numbers are more likely to be underpowered to detect real associations, but also to produce spurious positive or negative associations.Reference Berlin, Begg and Louis 57 However, this may not be a factor for the results among neonates, which are consistent.
The relationship between birth weight and BP among adolescents has been reported in previous reviews as either inconsistentReference Law and Shiell 12 or inverse with smaller effects than observed among (prepubescent) children.Reference Huxley, Shiell and Law 13 Similarly, this review found an inconsistent relationship between birth weight and BP among adolescents, while the relationship among younger children was generally inverse. The positive relationship observed in neonates is as expected, with the duration between birth and BP assessment too short to allow for any impact of subsequent weight trajectory. Explanations for the changing relationships among children and adolescents are uncertain, but could possibly relate to different growth patterns, for example, catch-up growth among those of low birth weight, and hormonal changes occurring at adolescence.Reference Law and Shiell 12 , Reference Ewald and Haldeman 58
Adjustment for possible confounding factors varied and was often incomplete. Consistent with previous reviews, which mainly included papers from HICs, studies adjusting for current body size (weight and, or, height) were more likely to report an inverse relationshipReference Hardy, Sovio and King 59 , Reference Huxley, Neil and Collins 60 than those that did not make such an adjustment. Adjusting for current size has been noted to lead to a stronger inverse relationship between birth weight and BP compared with results without such adjustments.Reference Blake, Gurrin and Evans 6 In most of the reviewed papers, that reported estimates adjusted for current size, unadjusted estimates for the effect of birth weight on BP were not reported. Therefore, we were unable to establish whether adjusting for current weight leads to stronger inverse relationships in these populations. The interpretation of findings adjusting for current weight is complexReference Blake, Gurrin and Evans 6 , Reference Hardy, Sovio and King 59 because current weight may be seen as a confounder or mediator of the effect of birth weight on BP.Reference Blake, Gurrin and Evans 6
Several mechanisms such as obesity, salt-sensitivity, renin–angiotensin system and endothelial activation are important in the pathophysiology of hypertension. None of the reviewed papers investigated the role of these factors and their impact on BP. Recent evidence suggests that the relationship between birth weight and BP could be U shaped,Reference Curhan, Willett and Rimm 61 highlighting the importance of both reduced and excessive nutrition in utero. It was not possible to examine this hypothesis from the papers reviewed. Compared with birth weight, other measures such as birth body mass index or pendular index may more accurately reflect the birth size, but none of the studies reviewed included these measures.
Studies were subject to selection bias as individuals most likely to be low birth weight (such as preterm and twins) were excluded in many studies. This could have led to an underestimation of the effect of birth weight on BP. Furthermore, characteristics [such as maternal hypertension, parasitic infections, socioeconomic status (past or current)] that influence birth weight and may also be associated with BP in offspring were not adjusted for in these studies. Hence, estimates were subject to residual confounding. It remains uncertain what role (if any) such factors have in the relationship between birth weight and subsequent BP in children or adolescents.
Inconsistencies seen between reviewed papers are less likely to be due to differences in BP measurement procedures, as studies followed a similar approach. For nearly all papers, there was an initial rest period (before starting BP procedure) and between successive BP measurements, automated devices were used and analysis was based on the average of two or three measurements. In the majority of studies, early life information including birth weight was prospectively collected thus studies were less prone to misclassification and recall bias.
Generally, studies reviewed came from all the African regions but with a strong representation of West Africa and Southern Africa. East Africa and North Africa had the least number of papers (one each) reviewed. The only paper from East Africa reported on an island population, thus there were no results on the mainland population of East Africa.
In conclusion, relatively few studies have investigated the relationship between birth weight and BP later in life in Africa. The relationship between birth weight and BP varied depending on the age of the participants. Our review emphasizes the need for larger studies on the relationship between birth weight and later BP from Africa, applying appropriate control of potential confounding factors. Accumulating evidence on raised BP in Africa and understanding the impact of growth in early life and of prenatal exposures on BP later in life is key in identifying at-risk groups and developing early life interventions to reduce BP risk in later life.
Acknowledgements
None.
Ethical Standards
Review was based on published manuscripts thus ethical approvals were not required.
Financial Support
This work was supported with funding from the Commonwealth Scholarship Commission (S.L., PhD funding at the LSHTM); the Wellcome Trust (A.E., grant number 095778) (L.S., grant number 098504/Z/12/Z); and the UK Medical Research Council (E.W., grant number MR/K012126/1).
Conflicts of Interest
None.






