Introduction
Psychotic disorders are the most severe and disabling types of mental disorders, characterized by the inability to distinguish the internal experience of the mind from the external reality of one's surroundings (Lieberman & First, Reference Lieberman and First2018; Owen, Sawa, & Mortensen, Reference Owen, Sawa and Mortensen2016). Schizophrenia, the most common type of psychotic disorder, includes a diverse set of symptoms including delusions, hallucinations, loss of contact with reality, loss of motivation, social withdrawal, and cognitive impairment (Lieberman & First, Reference Lieberman and First2018; Owen et al., Reference Owen, Sawa and Mortensen2016). Between 2.3% and 3.5% of people will experience some type of psychotic disorder in their lifetime (Owen et al., Reference Owen, Sawa and Mortensen2016). Most psychotic disorders first begin to develop in late adolescence and early adulthood (Kessler et al., Reference Kessler, Amminger, Aguilar-Gaxiola, Alonso, Lee and Ustün2007). Psychotic disorders are associated with many adverse outcomes including suicide, homelessness, unemployment, and an average life expectancy of 10–20 years less than the general population (Lieberman & First, Reference Lieberman and First2018; Owen et al., Reference Owen, Sawa and Mortensen2016).
Epidemiologic research suggests that cannabis use may be a significant risk factor for psychotic disorders. A meta-analysis of longitudinal studies estimated that lifetime cannabis users had an odds ratio of 2.58 (95% CI 1.08–6.13) for psychotic disorders compared to non-users (Moore et al., Reference Moore, Zammit, Lingford-Hughes, Barnes, Jones, Burke and Lewis2007). Another meta-analysis found an odds ratio of 3.90 (95% CI 2.84–5.34) for psychotic disorders among the most frequent cannabis users compared to non-users, suggesting dose–response (Marconi, Di Forti, Lewis, Murray, & Vassos, Reference Marconi, Di Forti, Lewis, Murray and Vassos2016). Whether cannabis use is causally related to psychotic disorders continues to be debated, with recent genetic studies raising uncertainty about the directionality of the relationship and the magnitude of association (Ganesh & D'Souza, Reference Ganesh and D'Souza2022; Gillespie & Kendler, Reference Gillespie and Kendler2021).
The link between cannabis use and psychotic disorders is biologically plausible. Experimental studies have found that cannabis intoxication can contribute to acute transient psychotic episodes (D'Souza et al., Reference D'Souza, Perry, MacDougall, Ammerman, Cooper, Wu and Krystal2004; Ganesh et al., Reference Ganesh, Cortes-Briones, Ranganathan, Radhakrishnan, Skosnik and D'Souza2020; Ganesh & D'Souza, Reference Ganesh and D'Souza2022). Youth has been identified as a potentially vulnerable time to use cannabis as the brain is still developing (Lubman, Cheetham, & Yücel, Reference Lubman, Cheetham and Yücel2015). Cannabis use during this formative period is suspected to impact the endocannabinoid system in a way that disrupts synaptic refinement, white matter development, and CB1 receptor binding (Lubman et al., Reference Lubman, Cheetham and Yücel2015). The main psychoactive ingredient of Δ9-tetrahydrocannabinol (THC) is thought to explain this relationship (Lubman et al., Reference Lubman, Cheetham and Yücel2015).
While the relationship between youth cannabis use and psychotic disorders is biologically plausible and supported by epidemiologic evidence to date, methodological limitations of previous studies make it difficult to estimate the strength of association. Most notably, the current evidence base of population-based cohort studies relies largely on cannabis exposure data from the 20th century when cannabis was significantly less potent (McDonald, Roerecke, & Mann, Reference McDonald, Roerecke and Mann2019). For example, the average THC potency of illicit herbal cannabis in Canada increased from less than 1% prior to 1980 to 6% in the late 1990s, 15% in 2016, and 20% in 2018 (Mahamad, Wadsworth, Rynard, Goodman, & Hammond, Reference Mahamad, Wadsworth, Rynard, Goodman and Hammond2020; McDonald, Kurdyak, Rehm, Roerecke, & Bondy, Reference McDonald, Kurdyak, Rehm, Roerecke and Bondy2024). New types of cannabis products have also become more popular including cannabis extracts, which can reach upwards of 95% THC (Smart, Caulkins, Kilmer, Davenport, & Midgette, Reference Smart, Caulkins, Kilmer, Davenport and Midgette2017). It is therefore possible that the strength of association between cannabis use and psychotic disorders has increased as a result of increasing cannabis potency (Hjorthøj, Posselt, & Nordentoft, Reference Hjorthøj, Posselt and Nordentoft2021). Moreover, due to the low incidence of psychotic disorders, few population-based cohort studies have had sufficient sample size to use a clinical diagnosis outcome (Arseneault et al., Reference Arseneault, Cannon, Poulton, Murray, Caspi and Moffitt2002; Hjorthøj et al., Reference Hjorthøj, Compton, Starzer, Nordholm, Einstein, Erlangsen and Han2023; Mustonen et al., Reference Mustonen, Niemelä, Nordström, Murray, Mäki, Jääskeläinen and Miettunen2018; Myran et al., Reference Myran, Harrison, Pugliese, Solmi, Anderson, Fiedorowicz and Tanuseputro2023; Zammit, Allebeck, Andreasson, Lundberg, & Lewis, Reference Zammit, Allebeck, Andreasson, Lundberg and Lewis2002), which is more relevant to public health than symptom- or experience-based outcomes employed by most previous studies (Gage, Zammit, & Hickman, Reference Gage, Zammit and Hickman2013).
Understanding the relationship between youth cannabis use and psychotic disorders is a critical public health issue (Ganesh & D'Souza, Reference Ganesh and D'Souza2022), especially as more jurisdictions liberalize cannabis use and perception of harm declines among youth (Mennis, McKeon, & Stahler, Reference Mennis, McKeon and Stahler2022). The objective of this study was to estimate the association between cannabis use during youth and risk of psychotic disorder diagnosis using recent population-based data.
Methods
Data sources
This study used Ontario data from the 2009 to 2012 cycles of the Canadian Community Health Survey (CCHS) linked to administrative health data at the Institute for Clinical Evaluative Sciences (ICES). The CCHS is an annual cross-sectional survey that collects information on health status, health care use, and social determinants of health within the Canadian population (Statistics Canada, 2013). Administrative health data housed at ICES included: hospitalization data from the Discharge Abstract Database (DAD) and the Ontario Mental Health Reporting System (OMHRS); ambulatory visit data from the National Ambulatory Care Reporting System (NACRS); physician billing data from the Ontario Health Insurance Plan (OHIP); and date of death and demographic data from the Registered Persons Database (RPDB). These datasets were linked using unique encoded identifiers and analyzed at ICES.
Study cohort
The source population for this study was non-institutionalized Ontario residents between the ages of 12 and 24 years who completed the CCHS from 2009 to 2012. The CCHS sampling frame covered ~98% of the Canadian population aged 12 and older, excluding residents in foster care, in the Canadian Forces, and living on reserves or other Indigenous settlements (Statistics Canada, 2013). Across the included CCHS cycles, approximately three-quarters of respondents had exclusively in-person interviews while others either participated by phone or both in-person and phone. The Canada-level combined (household and person) response rates for the CCHS cycles were 72.3% for 2010–11 and 66.4% for 2011–12. The survey was designed to ensure over-representation of youth aged 12 to 19 years (Statistics Canada, 2013).
Exclusions
We excluded respondents who used health services for psychotic disorders in the 6 years prior to their CCHS interview date to mitigate risk of reverse causation. We excluded individuals whose health records were not linkable, who were not registered with OHIP at baseline or for 180 consecutive days or more in the 2 years prior to CCHS, or whose self-reported sex or age in the CCHS did not match their corresponding RPDB record. For respondents who responded to more than one cycle of the CCHS, we used only their first interview. We excluded interviews completed by a proxy (due to mental/physical health problem that made it impossible for the selected youth to complete the interview during the collection period) and those who refused to answer the cannabis question. As shown in Fig. 1, after exclusions the study had a final unweighted sample size of n = 11 363.
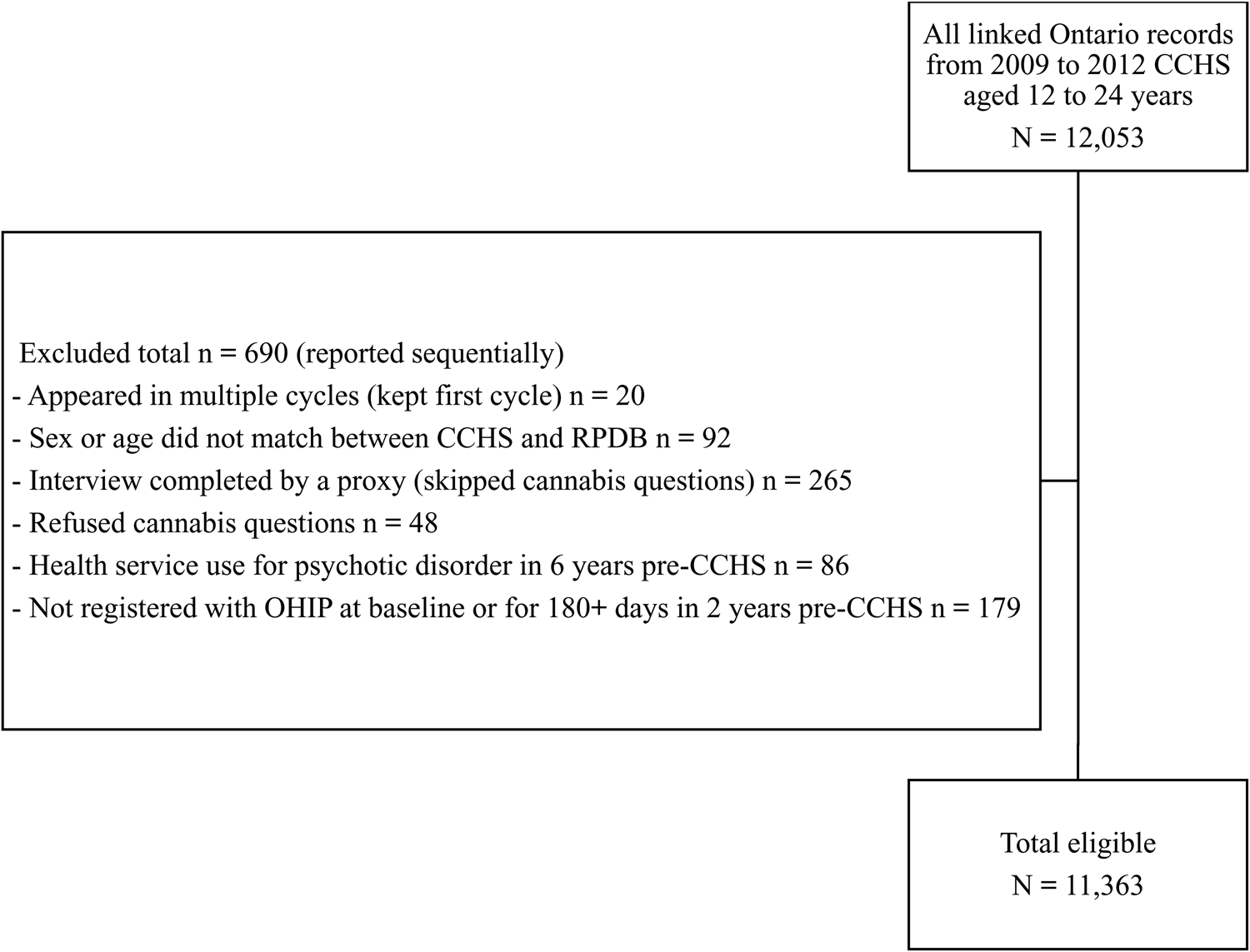
Figure 1. Flowchart of study sample exclusions.
Longitudinal design
We followed respondents from their CCHS interview until 2018 (the year Canada legalized recreational cannabis use). Thus, maximum follow-up time was 6 to 9 years depending on the survey cycle – i.e. 2012 respondents had 6-year follow-up and 2009 respondents had 9-year follow-up maximum. Research suggests that there is an average of 7 to 8 years between cannabis use initiation and onset of psychotic symptoms (Stefanis et al., Reference Stefanis, Dragovic, Power, Jablensky, Castle and Morgan2013), and an average delay of 1 to 2 years between onset of symptoms and treatment-seeking (Lieberman & Fenton, Reference Lieberman and Fenton2000). We selected a long follow-up period to reflect the typically long induction and latency periods between cannabis exposure and psychotic disorder treatment-seeking. We followed respondents from CCHS interview date to outcome or censoring at the end of follow-up. We defined end of follow-up as the earliest of respondent death, ceasing to have health insurance coverage (defined as the start of 90 consecutive unregistered days), or the end of the 6- to 9-year follow-up window. Thus, the earliest possible lookback date to establish pre-CCHS psychotic disorder health service use was 1 January 2003 and the latest possible follow-up date was 31 December 2018.
Exposure
Cannabis use was measured with the following question: ‘I am going to ask some questions about drug use. Again, I would like to remind you that everything you say will remain strictly confidential. Have you ever used or tried marijuana, cannabis or hashish? Yes, just once; Yes, more than once; No; Don't know; Refuse.’ If the respondent answered yes, they were then asked: ‘Have you used it in the past 12 months? Yes; No; Don't know; Refuse.’ We dichotomized past-year cannabis use as yes or no.
Outcome
The primary outcome was days to first outpatient physician visit, ED visit, or hospital discharge related to a psychotic disorder according to corresponding diagnostic codes. We used a validated algorithm (Kurdyak, Lin, Green, & Vigod, Reference Kurdyak, Lin, Green and Vigod2015) to identify individuals who met criteria for a psychotic disorder in the lookback and follow-up periods based on pre-established diagnostic codes (see eTable 1 in supplemental materials for list of codes). Our outcome did not include acute cannabis-induced or other substance-induced psychotic disorder hospitalizations or ED visits. Instead, our outcome was designed to identify the onset of chronic psychotic illness (Kurdyak et al., Reference Kurdyak, Lin, Green and Vigod2015).
Confounders
We used a directed acyclic graph to identify a minimal sufficient adjustment set of confounders based on previous literature. Sociodemographic confounders included self-reported assigned sex (female or male; gender not measured), baseline age (12 to 24 years), race (white or non-white), household income (<$ 50 000, $ 50 000–$ 99 999, $ 100 000+, or unknown), and rurality (rural or urban). For those under 18 years of age, the person most knowledgeable reported household income (Statistics Canada, 2013). From 2011 onward, Statistics Canada imputed missing household income data (Statistics Canada, 2013). We coded race as white or non-white due to low frequencies for certain non-white races and to mask Indigenous identity, which would have been inappropriate to identify in the absence of community engagement.
Substance use confounders included alcohol use in the past 12 months (yes or no), smoking in the past 12 months (yes or no), and illicit drug use in the past 12 months (yes or no). Illicit drug use was measured by asking a series of questions about different types of drugs including cocaine, ecstasy, hallucinogens, heroin, inhalants, and stimulants. Questions included: ‘Have you ever used or tried cocaine or crack? Have you ever used or tried ecstasy (MDMA) or other similar drugs? Have you ever used or tried hallucinogens, PCP or LSD (acid)? Have you ever used or tried heroin? Did you ever sniff glue, gasoline or other solvents? Have you ever used or tried speed (amphetamines)?’ Answering yes to any of these questions and a follow-up question asking if it had been in the past 12 months indicated illicit drug use in the past 12 months. Unmeasured confounders that we could not adjust for included trauma, genetic predisposition, and family history of psychotic disorders.
Main analysis
We pooled data and combined survey weights from the 2009 to 2012 cycles of the CCHS. We used a multivariable Cox proportional hazards model to estimate the adjusted hazard ratio (aHR) for the association between cannabis use and risk of psychotic disorder. We used age as the time scale with delayed entry to account for left truncation and included baseline age as a covariate (Jin, Ton, Incerti, & Hu, Reference Jin, Ton, Incerti and Hu2023; Pencina, Larson, & D'Agostino, Reference Pencina, Larson and D'Agostino2007). We used Efron approximation and excluded records with missing data listwise (n = 159). We examined multicollinearity with variance inflation factors and assessed the proportional hazards assumption with Kaplan–Meier curves and Schoenfeld residuals. We tested covariate × time interactions where non-proportionality was suggested.
Sensitivity analyses
We re-ran the main analysis Cox model under the following conditions to examine the robustness of our focal estimate to potential sources of bias and different modeling approaches:
1. No adjustment.
2. Adjusting for sociodemographic confounders only.
3. Excluding respondents aged 12–13 years (before most youth initiate cannabis use).
4. Excluding former cannabis users (lifetime but not in past year) from reference group.
5. Using lifetime cannabis use instead of past-year use as the focal exposure.
6. Ignoring all lookback exclusions to examine impact of left truncation.
7. Maximum 3 years of follow-up to minimize potential exposure misclassification.
8. Outcome restricted to hospitalizations/ED visits to increase specificity (Kurdyak et al., Reference Kurdyak, Lin, Green and Vigod2015).
We calculated the E value for the lower 95% confidence limit of the focal association from the main analysis. The E value is defined as the minimum strength of association that an unmeasured confounder would need to have with both the exposure and the outcome to fully explain away a specific exposure-outcome association, conditional on the measured covariates (19).
We conducted follow-up analyses to explore dose–response, sex differences, and reverse causation. For dose–response, we replicated the main analysis model but with past-year cannabis use frequency (never, <weekly, and weekly+) as the focal exposure. For sex differences, we replicated the main analysis model but with a cannabis × sex interaction. As these exploratory Cox models were likely to be underpowered, we conducted them with and without survey weight adjustment and bootstrap variance estimation to ensure stable estimates. To explore reverse causation, we conducted a multivariable modified Poisson model (Zou, Reference Zou2004) with previous health service use for psychotic disorders (in the 6 years pre-CCHS) as the exposure and past-year cannabis use reported in the CCHS as the outcome, adjusting for the same covariates and treating age as continuous.
In accordance with Statistics Canada guidelines, all statistical analyses incorporated survey weights for point estimation and bootstrap weights for variance, p value, and confidence interval estimation (Statistics Canada, 2013). We conducted analyses using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC) and Stata/MP 15.1 for Unix (StataCorp, College Station, TX) and reported our findings in accordance with STROBE guidelines. p < 0.05 and 95% confidence intervals that did not include the null were considered statistically significant.
Results
Table 1 presents baseline characteristics of the eligible study cohort stratified by past-year cannabis use. Overall, 23.4% of respondents reported cannabis use in the past year. In total, 1.2% of respondents used health services for psychotic disorders during follow-up and 4.0% were right censored due to loss of health insurance registration or death.
Table 1. Baseline characteristics (weighted) of the pooled study sample (N = 11 363)
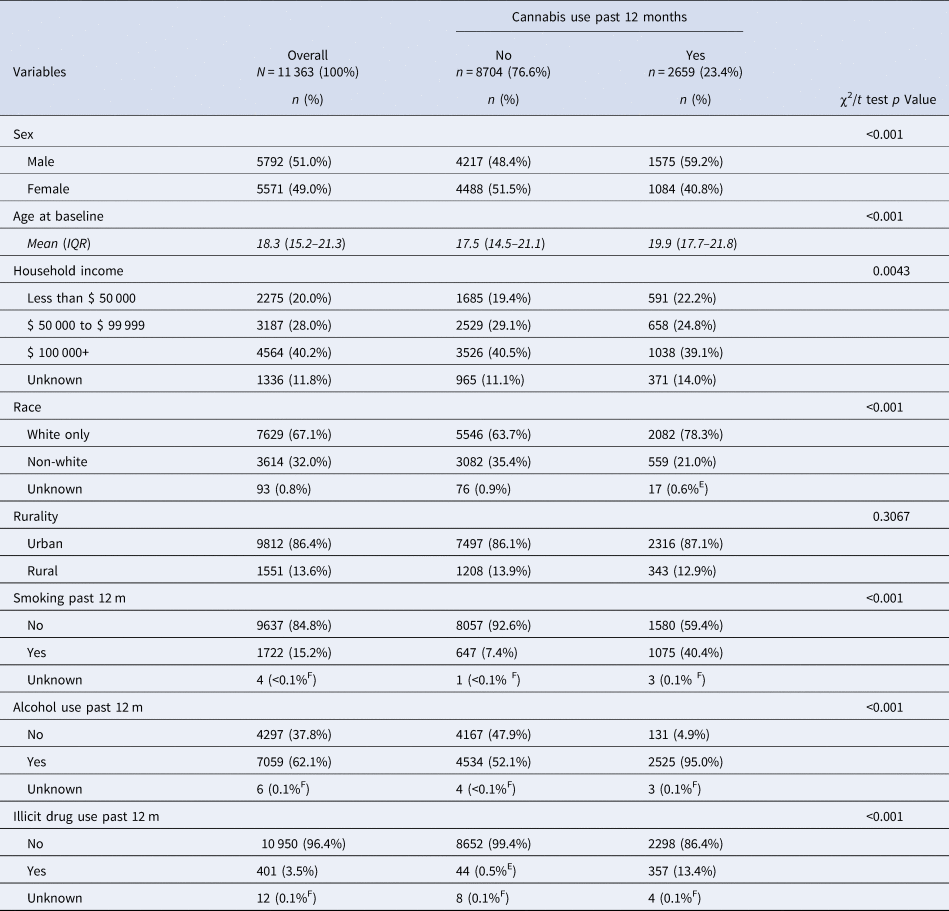
Notes: IQR, interquartile range. Frequencies were estimated using survey weights normalized to the final eligible sample size. Discrepant totals are due to rounding of weighted frequencies and percentages. All reported percentages had a coefficient of variation (CV) under 0.166 in accordance with Statistics Canada reporting guidelines unless indicated by:
E, high sampling variability (0.166 < CV ⩽ 0.334); caution should be used in interpreting this estimate. F, extreme sampling variability (CV > 0.333); estimate is unreliable.
The proportional hazards assumption is a key assumption for Cox models, requiring that risk of the outcome in one group relative to the other group(s) is constant over time (Kleinbaum & Klein, Reference Kleinbaum, Klein, Kleinbaum and Klein2012). We detected possible violation of this assumption for cannabis use, income, smoking, and baseline age. This study used age as the time scale, therefore we tested covariate × age–time interactions to determine whether the proportional hazards assumption held. Income × age–time and smoking × age–time interactions were not statistically significant (p > 0.05), but cannabis × age–time was statistically significant (p < 0.05), suggesting that the association between cannabis and psychotic disorders was age-dependent. We removed baseline age as a covariate from the model because it was already accounted for in the time scale, which changed the focal point estimate negligibly (<0.1 on HR scale). Cumulative incidence curves showed that the association between cannabis use and psychotic disorders had an inflection point at 20 years of age–time (see Fig. 2). To model this relationship according to best practices (Kleinbaum & Klein, Reference Kleinbaum, Klein, Kleinbaum and Klein2012), we used an extended Cox model that included interactions between cannabis use and Heaviside functions of age–time (above and below 20 years of age-time) to estimate aHRs for both intervals of age–time during which the proportional hazards assumption was met.
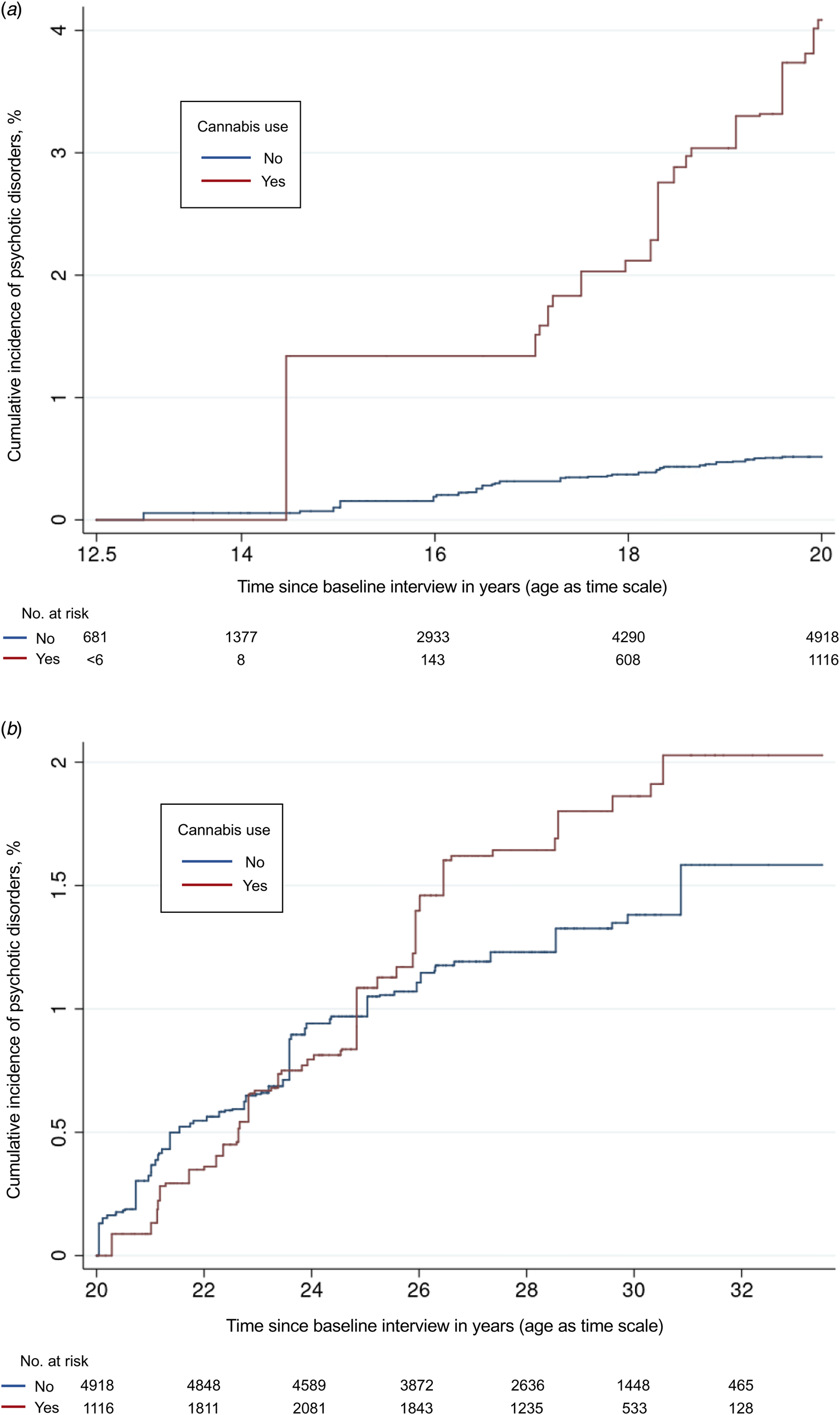
Figure 2. (a) Cumulative incidence of psychotic disorders stratified by cannabis use from 12 to 19 years of age. (b) Cumulative incidence of psychotic disorders stratified by baseline cannabis use from 20 to 33 years of age.
Notes: Cumulative incidence curves are weighted based on survey weights. We added half a year to respondents' baseline age to reflect that interviews were conducted throughout the calendar year and not on respondents' birthdays. Instability on the left side of Fig. 2a was due to the relatively small number of cannabis users under 15 years of age; however, the risk set was sufficiently large for a reliable product-limit estimator in the presence of left truncation and right censoring (Lai & Ying, Reference Lai and Ying1991). Numbers at risk were calculated using normalized survey weights and increase at first due to the delayed entry of respondents.
Table 2 shows results from the multivariable extended Cox model. Past-year cannabis use was significantly associated with psychotic disorders from 12 to 19 years of age-time (aHR = 11.21; 95% CI 4.60–27.33) but not from 20 to 33 years of age–time (aHR = 1.29; 95% CI 0.63–2.64).
Table 2. Multivariable extended Cox proportional hazards model for psychotic disorders (n = 11 204)
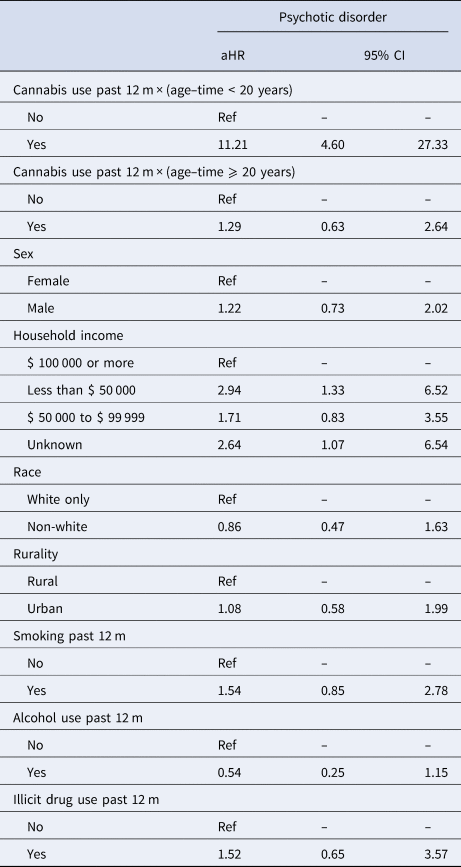
aHR, adjusted hazard ratio; 95% CI, 95% confidence interval; Ref, reference group.
Adjusted hazard ratios for the association between cannabis use and psychotic disorders conditional on attained age–time were estimated using interactions between past-year cannabis use and Heaviside functions of age–time (above 20 years and below 20 years).
Table 3 shows results from the sensitivity analyses. Our focal estimates were robust to many different model conditions and did not change meaningfully in terms of statistical significance. When we restricted the outcome to hospitalizations/ED visits, the strength of association for adolescent cannabis use increased markedly (aHR = 26.68; 95% CI 7.67–92.76). We probed this strong association and found that of all the incident psychotic disorder hospitalizations/ED visits during adolescence, 77.8% (95% CI 56.4–99.3%) had reported past-year cannabis use at baseline and 82.3% (95% CI 64.7–100.0%) had reported lifetime use.
Table 3. Sensitivity analyses for the association between cannabis use and psychotic disorders conditional on attained age-time
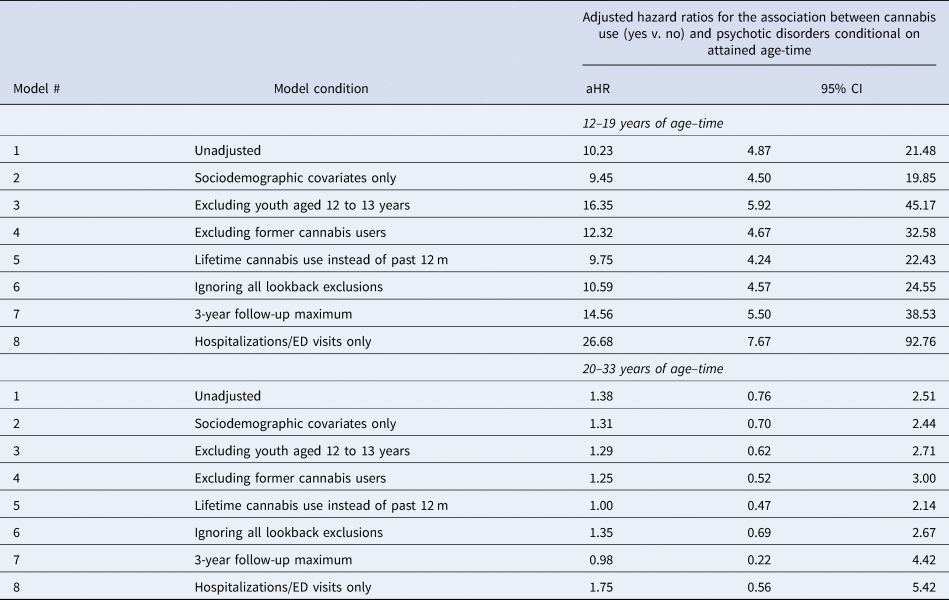
Like the main analysis, sensitivity analyses were estimated using multivariable extended Cox modeling with interactions between cannabis use and heaviside functions of age–time above and below 20 years. Unweighted Ns for the eight models (in order): 11 363; 11 232; 9431; 9566; 11 209; 11 430, 11 204; and 11 204. Estimates from the eight models were stratified by age–time to facilitate comparison.
The E value for the lower 95% confidence limit from the main analysis for adolescent cannabis use was E = 8.7, suggesting that the confidence interval could be moved to include the null by an unmeasured confounder that was associated with both cannabis use and psychotic disorders by a hazard ratio of 8.7-fold each, above and beyond the measured confounders, but weaker confounding could not do so (VanderWeele & Ding, Reference VanderWeele and Ding2017).
Results from the follow-up analyses are presented in eTable 2 in the supplemental materials. We re-ran the main analysis with cannabis use frequency as the focal exposure to explore dose–response. In the weighted model, weekly or more cannabis use during adolescence was the only statistically significant estimate (aHR = 10.70; 95% CI 3.49–32.78). In the unweighted model, less than weekly and weekly or more cannabis use were associated with psychotic disorders both during adolescence and early adulthood, though we only observed a gradient during adolescence with the strongest association for weekly or more cannabis use. Using a model that included a non-significant cannabis × sex × age–time interaction (p > 0.05), we estimated sex-specific hazard ratios. In the weighted model, the effect of cannabis was only statistically significant for males during adolescence (aHR = 9.98; 95% CI 2.89–34.47). In the unweighted model, cannabis use was significantly associated with psychotic disorders for males during both adolescence and early adulthood and among females only during adolescence. When exploring reverse causation with a multivariable modified Poisson model, we found that using health services for a psychotic disorder in the 6 years prior to CCHS interview was significantly associated with reporting past-year cannabis use during the CCHS interview (adjusted risk ratio = 1.41; 95% CI 1.02–1.94).
Discussion
We found that cannabis use, compared to no cannabis use, was associated with over 11 times (95% CI 4.6–27.3) greater risk of psychotic disorder at any point during adolescence (ages 12–19 years). We found no evidence of association between cannabis use and risk of psychotic disorder during young adulthood (ages 20–33 years). Many have hypothesized that adolescence is a more sensitive risk period than adulthood for the effect of cannabis use on psychotic disorder development, yet prior to this study, little epidemiologic evidence existed to support this view (Gage, Hickman, & Zammit, Reference Gage, Hickman and Zammit2016; Lawn et al., Reference Lawn, Mokrysz, Lees, Trinci, Petrilli, Skumlien and Curran2022). Some studies have found that cannabis use disorder is most strongly associated with schizophrenia in adolescent males compared to other age by sex subgroups (Hjorthøj et al., Reference Hjorthøj, Compton, Starzer, Nordholm, Einstein, Erlangsen and Han2023; Myran et al., Reference Myran, Harrison, Pugliese, Solmi, Anderson, Fiedorowicz and Tanuseputro2023), but other studies have reported that the association between cannabis use and psychotic symptoms either only becomes apparent in early adulthood or is no different in adolescence compared to early adulthood (Lawn et al., Reference Lawn, Mokrysz, Lees, Trinci, Petrilli, Skumlien and Curran2022; Leadbeater, Ames, & Linden-Carmichael, Reference Leadbeater, Ames and Linden-Carmichael2019). This study therefore provides important new epidemiologic evidence consistent with the neurodevelopmental theory that adolescence is a particularly vulnerable time to use cannabis.
We observed a stronger measure of association during adolescence than the vast majority of previous studies. Meta-analyses of longitudinal studies suggest that cannabis use roughly doubles the risk of developing a psychotic disorder compared to non-users (Gage et al., Reference Gage, Hickman and Zammit2016; Kiburi, Molebatsi, Ntlantsana, & Lynskey, Reference Kiburi, Molebatsi, Ntlantsana and Lynskey2021; Moore et al., Reference Moore, Zammit, Lingford-Hughes, Barnes, Jones, Burke and Lewis2007). Key differences that could help explain this discrepancy included our use of a clinical outcome, our use of more recent data, and our ability to identify an age-dependent association.
Most previous cohort studies have examined less severe psychotic experiences rather than psychotic disorders (Gage et al., Reference Gage, Hickman and Zammit2016; Kiburi et al., Reference Kiburi, Molebatsi, Ntlantsana and Lynskey2021), likely because they are much more common and therefore better suited to a longitudinal design. However, meta-analyses suggest that cannabis use is more strongly associated with psychotic disorders than with psychotic experiences (Marconi et al., Reference Marconi, Di Forti, Lewis, Murray and Vassos2016; Moore et al., Reference Moore, Zammit, Lingford-Hughes, Barnes, Jones, Burke and Lewis2007). Our data also suggest that cannabis use is more strongly associated with more severe psychotic outcomes as the strength of association during adolescence increased markedly when we restricted the outcome to hospitalizations and ED visits (the most severe types of health service use). We highlight that of all the incident psychotic disorder hospitalizations/ED visits during adolescence, roughly 5 in 6 had reported lifetime cannabis use at baseline.
We used recent data when cannabis was on average more potent than previous cohort studies, which may also have contributed to the stronger measure of association. There is early evidence to support this explanation, with studies suggesting that the population-attributable risk fraction of cannabis use disorder in schizophrenia has increased over time due to increasing potency (Hjorthøj et al., Reference Hjorthøj, Posselt and Nordentoft2021), and that high-potency cannabis contributes significantly to variation in the incidence of psychotic disorders in Europe (Di Forti et al., Reference Di Forti, Quattrone, Freeman, Tripoli, Gayer-Anderson, Quigley and Group2019).
Our ability to identify an age-dependent association – because of the age range of our cohort, the time-to-event structure of our linked data, and the delayed entry design of our study – may be another contributor to the strong measure of association we observed during adolescence. Previous research has found that earlier cannabis use is more strongly associated with schizophrenia in adulthood (Arseneault et al., Reference Arseneault, Cannon, Poulton, Murray, Caspi and Moffitt2002), and that cannabis use is associated with earlier age of onset of psychosis (Large, Sharma, Compton, Slade, & Nielssen, Reference Large, Sharma, Compton, Slade and Nielssen2011). However, most previous population-based cohort studies have examined psychotic outcomes in adulthood, which may have missed a critical window of psychotic disorder development and masked the timing of a stronger association. Had we treated the association between cannabis use and psychotic disorders as invariant across the age–time continuum, our model would have produced an aHR of 2.19 (95% CI 1.11–4.31). We note that a linkage study from Finland used time-to-event registry data and found a hazard ratio for adolescent cannabis use that is more in line with previous research (Mustonen et al., Reference Mustonen, Niemelä, Nordström, Murray, Mäki, Jääskeläinen and Miettunen2018). However, unlike the current study, the Finnish study measured cannabis use in the early 2000s, only captured cannabis use at 15 or 16 years of age for all participants (before most youth initiate cannabis use), and followed participants until 30 years of age (i.e. 15 years of follow-up), likely contributing to greater misclassification of exposed person-time and making it difficult to observe an age-dependent association.
Sensitivity analyses suggested that our main analysis was robust to many different model conditions. Hazard ratio estimates did not change meaningfully in terms of statistical significance under any of these model conditions. In most of the models that controlled for the full set of confounding variables, aHR estimates for cannabis use during adolescence increased, suggesting that our main analysis estimate may have been conservative.
Unmeasured confounders and effect modifiers
It is unclear to what extent unmeasured confounders – including genetic predisposition, family history of psychotic disorders, and trauma – biased our results. Most notably, we had no way of assessing the potential confounding impact of genetic predisposition to psychotic disorders. There is a large body of research suggesting that psychotic disorders are heritable; yet evidence is mixed for whether genetic predisposition to psychotic disorders robustly predicts cannabis use. Some studies suggest that the association between cannabis and psychosis may be explained by shared genetic liability, while others suggest that only a small proportion of variance in cannabis use is explained by common genetic variants, or that genetic predisposition to psychotic disorders does not differ between cannabis users and non-users (Johnson et al., Reference Johnson, Hatoum, Deak, Polimanti, Murray, Edenberg and Agrawal2021). Altogether, it is likely that unmeasured confounding biased our results away from the null but based on our sensitivity analysis and previous literature it seems unlikely that confounding could explain away the association we observed.
Research also suggests that genetic predisposition and childhood trauma may moderate the association between cannabis use and psychotic disorders (Kiburi et al., Reference Kiburi, Molebatsi, Ntlantsana and Lynskey2021). If this were the case, we likely overestimated the strength of association for those without genetic predisposition and trauma history and underestimated the strength of association for those with genetic predisposition and trauma history.
Reverse causation
Reverse causation has been advanced as an alternative explanation for the association between youth cannabis use and psychotic disorders, where individuals with psychotic symptoms self-medicate with, or are predisposed to use, cannabis (Hall & Degenhardt, Reference Hall and Degenhardt2008). While the current study excluded respondents with prior health service use for psychotic disorders to mitigate risk of reverse causation, this exclusion criterion did not eliminate the possibility of reverse causation entirely, as youth with psychotic disorders may have begun using cannabis after the onset of prodromal symptoms but before seeking treatment. If there was a bidirectional relationship, a feedback loop between cannabis use and psychotic symptoms where each reinforced the other could have biased our focal estimates away from the null. This may have affected our estimate differentially for adolescents compared to young adults, as previous research suggests that adolescents have a longer average duration of untreated psychosis compared to adults (Dominguez et al., Reference Dominguez, Fisher, Major, Chisholm, Rahaman, Joyce and Hodes2013). Psychotic symptoms are more difficult to identity in adolescents, and may be misdiagnosed as emotional or behavioural problems by families and non-health professionals, which can delay referral to, and use of, mental health services (Dominguez et al., Reference Dominguez, Fisher, Major, Chisholm, Rahaman, Joyce and Hodes2013; Menezes & Milovan, Reference Menezes and Milovan2000). Thus, the age-dependent association we observed may have been influenced by important differences in the illness trajectories and health system interactions of adolescents and young adults. Recent genetic research, including Mendelian randomization studies, supports a bidirectional relationship between cannabis use and schizophrenia, with reverse causal mechanisms playing a stronger role in driving the association (D'Souza, Reference D'Souza2023). However, reverse causation and bidirectional hypotheses depend on whether psychotic symptoms lead to cannabis use, and evidence on the whole is still mixed in this regard (Gage et al., Reference Gage, Hickman and Zammit2016; Gillespie & Kendler, Reference Gillespie and Kendler2021; Johnson et al., Reference Johnson, Hatoum, Deak, Polimanti, Murray, Edenberg and Agrawal2021).
Strengths and limitations
This study had several strengths. Data were derived from the CCHS – a high-quality, representative general population survey – and from ICES, which captures all health service use delivered in Ontario's universal healthcare system. We used a validated health service use outcome (Kurdyak et al., Reference Kurdyak, Lin, Green and Vigod2015), which is more objective than survey-based interviews and more relevant to public health than psychotic symptoms or experiences. Most previous longitudinal studies reliant on follow-up surveys and continued voluntary participation suffer from attrition bias (Gage et al., Reference Gage, Hickman and Zammit2016), whereas this study used administrative data which had minimal attrition. Cannabis potency has increased markedly in recent years, limiting the generalizability of previous research which has largely used 20th century data (McDonald et al., Reference McDonald, Roerecke and Mann2019); this study used cannabis use data from as recently as 2012 and exposed person-time up to 2018. To date, this is one of the largest cohort studies examining cannabis and psychotic disorders in terms of sample size.
Despite many improvements on the existing evidence base, this study had its own set of limitations. As previously mentioned, we were unable to control for potentially significant unmeasured confounders, nor could we definitively establish temporality between exposure and outcome. This study only had a single baseline measurement of cannabis and other substance use, likely contributing to exposure misclassification and time-varying confounding bias; however, we note that the focal estimates did not change meaningfully when using a model with a 3-year follow-up maximum, which was less susceptible to these biases. Our study relied on self-reported cannabis use from when recreational cannabis use was illegal for all ages in Canada, which may have contributed to underreporting (Gage et al., Reference Gage, Hickman and Zammit2016). Our cannabis measure was crude, as the dataset did not capture important factors including THC potency, mode of use, product type, or cannabis dependence. Our sample did not capture institutionalized and homeless youth – groups at high risk of cannabis use and psychotic disorders. Because of the low incidence of psychotic disorders in adolescence, our estimates for cannabis use had wide confidence intervals, particularly when stratified by sex and cannabis use frequency in the follow-up analyses.
Conclusion
This study found a strong but age-dependent association between cannabis use and psychotic disorders, consistent with the theory that adolescence is a particularly vulnerable time to use cannabis as the brain is still developing. We observed a stronger measure of association during adolescence than previous research, possibly reflecting the rise of cannabis potency in recent years. It is important to acknowledge that this study was observational, had potentially significant unmeasured confounding bias, and was limited in its ability to rule out reverse causation. All these factors make it impossible for this study to establish causality. However, much like the early history of cigarettes and lung cancer (D'Souza, Reference D'Souza2023), since it is not possible to conduct randomized studies for ethical reasons (especially among adolescents), methodologically rigorous cohort studies offer the best evidence possible for policymakers to make informed decisions. Based on the precautionary principle, as more jurisdictions move to liberalize cannabis use and perception of harm declines among youth, this study suggests that evidence-based cannabis prevention strategies for adolescents are warranted. Further longitudinal studies using contemporary data with more sophisticated cannabis measurement and a more comprehensive set of baseline and time-varying confounders are needed to strengthen causal inference.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291724000990
Acknowledgments
The authors would like to acknowledge the enormous contributions of the late Dr Robert E. Mann who supervised this work. Parts of this material are based on data and/or information compiled and provided by the Ontario Ministry of Health and the Canadian Institute for Health Information. ICES is an independent, non-profit research institute funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). As a prescribed entity under Ontario's privacy legislation, ICES is authorized to collect and use health care data for the purposes of health system analysis, evaluation and decision support. Secure access to these data is governed by policies and procedures that are approved by the Information and Privacy Commissioner of Ontario. The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding or data sources. No endorsement by ICES, CIHI, the MOH or MLTC is intended or should be inferred. This project was approved by the Health Sciences Research Ethics Board at the University of Toronto (Protocol 00041834). Adapted from Statistics Canada, Canadian Community Health Survey, 2009–10 and 2011–12. This does not constitute an endorsement by Statistics Canada of this product. This document used data adapted from the Statistics Canada Postal CodeOM Conversion File, which is based on data licensed from Canada Post Corporation, and/or data adapted from the Ontario Ministry of Health Postal Code Conversion File, which contains data copied under license from ©Canada Post Corporation and Statistics Canada.
Data sharing
The dataset from this study is held securely in coded form at ICES. While legal data sharing agreements between ICES and data providers (e.g. healthcare organizations and government) prohibit ICES from making the dataset publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS (email: das@ices.on.ca). The full dataset creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Funding statement
This study was supported by the Centre for Addiction and Mental Health, the University of Toronto, and ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC).
Competing interests
None to declare.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.







