It is well known from clinical studies that severe chronic vitamin D deficiency in adults causes osteomalacia, a bone condition characterized by bone pain, muscle weakness and fracture (either microfractures or transcortical fractures)(Reference Cianferotti and Marcocci1). A number of articles have occasionally reported limited series of undiagnosed cases of osteomalacia in individuals with vitamin D deficiency due to either nutritional reasons (e.g. gastrointestinal disorder and/or bariatric surgery) or a total absence of sun exposure (e.g. veiled women)(Reference Holick, Binkley and Bischoff-Ferrari2, Reference Le Goaziou, Contardo and Dupraz3). When patients present with symptoms such as chronic bone pain, muscle weakness or fracture, osteomalacia is rarely suspected by the referring physician, and the lag between the development of symptoms and the vitamin D deficiency diagnosis may be long(Reference de Torrenté de la Jara, Pécoud and Favrat4). It is possible that less severe but chronic vitamin D deficiency may cause comparable symptoms, although the 25-hydroxyvitamin D (25(OH)D) level at which symptoms may arise is not precisely known and probably highly variable across individuals(Reference Cianferotti and Marcocci1). Additionally, vitamin D deficiency has been found to be associated with increased incidence of fractures, falls, neuromuscular and endocrinal impairments, and chronic pain in elderly populations(Reference Annweiler, Allali and Allain5–Reference Christakos and Deluca7). However, very few studies have been conducted in healthy younger adults regarding the possible association between 25(OH)D levels and negative health outcomes although a significant proportion have vitamin D deficiency(Reference Annweiler, Allali and Allain5, Reference Tangpricha, Pearce and Chen8–Reference Forrest and Stuhldreher11). These studies also found that the most deficient individuals had not been previously diagnosed. Other studies suggested that the delay in vitamin D deficiency diagnosis generates over-use of inappropriate health-care resources prior to the deficiency being diagnosed and treated(Reference de Torrenté de la Jara, Pécoud and Favrat4, Reference Nellen, Smulders and Jos Frissen12–Reference Fabbriciani, Pirro and Leli15). Based on these hypotheses, we could expect that in patients with no severe co-morbidities in a primary care setting, when vitamin D deficiency is diagnosed and treated by vitamin D supplementation, it is associated with a reduction in health-care use thereafter. The purpose of the present study was to analyse health-care use before and after a prescription of vitamin D supplementation following a 25(OH)D assay in a young to middle-aged population and to compare it with that in a non-supplemented group regarded as a reference group.
Experimental method
The French Health Insurance Funds
France has a publicly funded health system that systematically covers the population(16). Briefly, maternity/sickness and paternity insurance benefits are provided by the local Health Insurance Funds (Caisses Primaires d’Assurance Maladie) and 99·9 % of the French population is covered with a public insurance (i.e. 63 million individuals). Reimbursements relate to all medical procedures given to the patients: visits to health-care professionals, drugs, biological and medical procedures.
Drug reimbursement in the French Health Insurance Funds
France has still a pharmaceutical monopoly; most drugs are only available in pharmacies (community or hospital), with a mandatory medical prescription. Drugs containing high doses of vitamin D (e.g. 2500 µg (100 000 IU) ampoules) must be prescribed before dispensation and are reimbursed on a 65 % regular basis.
Study design and study population
We conducted a before–after analysis of health-care use in a cohort of individuals who had a 25(OH)D assay and a vitamin D prescription after the assay and compared it with that in the reference group of individuals who did not have a vitamin D prescription after the assay. We used data from the Rhône-Alpes area (Reference Annweiler, Schott and Berrut6 million inhabitants in 2009) provided by the ERASME database (Extraction, Recherches, Analyses pour un Suivi Médico-Economique)(Reference Fender and Weill17), a regional component of the French Health Insurance Funds (FHIF).
Patients aged 13–60 years who had a 25(OH)D assay between 1 December 2008 and 31 January 2009 were selected. Patients presenting with one of the thirty severe chronic diseases classified as ‘affection de longue durée’ (ALD)(Reference Nolte, Knai and McKee18) were excluded, because people suffering severe chronic diseases are different from the general population regarding their health-care use. The list of diseases giving access to the ALD programme is currently defined. These chronic diseases include: stroke, bone marrow failure and other chronic cytopenia, chronic arteriopathy with ischaemic manifestations, complicated bilharziasis, severe heart diseases, chronic active liver disease and cirrhosis, primary or HIV acquired immunodeficiency, type I or II diabetes, severe neuromuscular disease (including myopathy and severe epilepsy), severe and chronic haemoglobinopathy, haemophilia, severe arterial hypertension, CHD, chronic respiratory failure, Alzheimer’s disease, Parkinson’s disease, metabolic inherited disease, cystic fibrosis, severe and chronic renal disease, paraplegia, vasculitides, systemic lupus erythematosus, scleroderma, rheumatoid arthritis, long-term psychiatric disease, chronic ulcerative colitis and Crohn’s disease, multiple sclerosis, evolutive scoliosis, severe spondylarthritis, organ transplant consequences, tuberculosis, leprosy and cancer diseases. This programme involves about 16 % of the patients insured by the FHIF. Patients with a vitamin D dispensation during the 5-month period preceding the assay, who died, or who were assigned to another health-care insurance system during the follow-up period were also excluded.
Outcome and factors associated with vitamin D deficiency
Individuals were considered as vitamin D deficient if they had a record of 25(OH)D assay followed by vitamin D supplementation (i.e. at least one occurrence of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) reimbursement over the 3 months after the assay). They are referred to as the supplemented group. Patients who were not prescribed vitamin D after their assay were considered as not 25(OH)D deficient, and were regarded as the reference group. Each patient of the reference group was matched with a patient of the supplemented group on 25(OH)D assay date, within a range of 5 d. The index date for each pair was the date of supplementation for the supplemented patient. Health-care use for every patient was recorded over two 5-month periods. Five months was chosen for the following reasons. First, the French Insurance Healthcare database is subject to regular turnover; only 24 monthsʼ data are stored, and each month, when the data of the newest month are entered in the system, those of the oldest month disappear. Second, we did not include in the analysis the period between the assay and the first prescription of vitamin D because no change in health-care use was expected during this period. Third, the last three months of the 24-month database is not as reliable as the preceding months because the data regarding prescriptions are entered in the system only at the time of the reimbursement. Although for most patients the reimbursement is done automatically when purchasing drugs with their health insurance card (carte ‘VITAL’) from the pharmacy, a minority (those who do not have their card when purchasing the drugs) do not appear in the database until they send their claims by mail. For comparing health-care use between pre- and post-supplementation periods, we extracted the following information from the database: age, sex, number of physician visits and recorded medical interventions (according to the French classification of medical procedures), different drug prescriptions, drug classes (defined by Anatomical Therapeutical Chemical (ATC) codes(19)) per distinct dates of prescription, medical imaging examinations, incident sick leaves and hospitalizations. The medical procedures were also classified as therapeutic or diagnostic. We identified and extracted reimbursements for specific drugs or biological examinations that were expected to be prescribed in patients presenting with vitamin D deficiency symptoms, such as diffuse musculoskeletal pain, asthenia or apparent depression. Regarding drugs, we considered analgesics of the second and third rung of the WHO’s Pain Relief Ladder (PRL)(20, Reference Vargas-Schaffer21), muscle relaxants, corticosteroids, thyroid hormones, iron-based preparations and antidepressants. Analgesics corresponding to the first rung of the PRL were not considered as they are too highly prone to over-the-counter use, and consequently estimation of their use would be strongly biased. Antiepileptic drugs were abstracted as they may be responsible for a vitamin D deficiency. We analysed claims for the following biological examinations: cell blood counts, transaminase level, serum creatinine and thyroid-stimulating hormone level, since they are expected to be prescribed in the case of vitamin D deficiency symptoms such as unspecific musculoskeletal pain.
Statistical analysis
The study population was described using means and standard deviations for continuous variables and frequencies and proportions for discrete variables. The supplemented and the reference groups were compared using the t test when variables were continuous and the χ 2 test when variables were discrete. Health-care use within each group was compared before and after the index date using a paired t test on the same sample for continuous variables, McNemarʼs χ 2 test for binomial discrete variables and Bowker’s test for discrete variables with more than two categories. A P value of 0·05 or less was considered statistically significant. Analyses were performed using the SAS Enterprise Guide version 4·3.
Results
Characteristics of the supplemented group
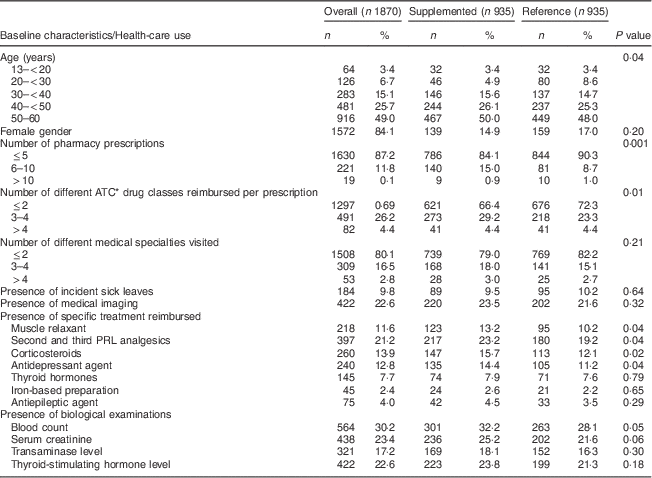
Over the two-month inclusion period (1 December 2008–31 January 2009), 3023 patients aged 13–60 years were identified (Fig. 1). Their mean age was 47·5 (sd 11·0) years, their median age was 49·8 years and 84·1 % were women. The first assay recorded was a 25(OH)D3 assay in almost all cases (98·5 %). Among these patients, 45·2 % (n 1367) received vitamin D supplementation following the assay and 935 of them were matched to the 935 controls who did not receive any 25(OH)D during the whole follow-up period. The mean age of the supplemented population was 46·9 (sd 10·8) years, their median age was 50·0 years and 85·1 % were women. Baseline characteristics are shown in Table 1. Half of the patients were 50–60 years old, 26·1 % were 40–50 years old and 23·9 % were 13–40 years old. Most patients (66·4 %) had up to two different classes of drugs prescribed over the 5-month baseline period before the 25(OH)D assay and 9·5 % had at least one sick leave. Thirteen per cent of patients were prescribed muscle relaxants, 15·7 % corticosteroids, and 23·2 % a second or third PRL analgesic.

Fig. 1 Flowchart of the study (ERASME, Extraction, Recherches, Analyses pour un Suivi Médico-Economique (database); ALD, ‘affection de longue durée’; 25(OH)D, 25-hydroxyvitamin D)
Table 1 Baseline characteristics of the study population and health-care use during the 5-month period preceding the 25(OH)D assay, overall and by further vitamin D supplementation status; data on young to middle-aged patients who had a 25(OH)D assay between 1 December 2008 and 31 January 2009, ERASME database (regional component of the FHIF), Rhône-Alpes area, France

25(OH)D, 25-hydroxyvitamin D; ERASME, Extraction, Recherches, Analyses pour un Suivi Médico-Economique; FHIF, French Health Insurance Funds; PRL, WHO’s Pain Relief Ladder.
*Anatomical Therapeutic Chemical classification of drugs.
Characteristics of the reference group
Baseline characteristics of the reference group are shown Table 1. The supplemented group had higher baseline health-care use compared with the reference group as seen by the number of distinct pharmacy prescriptions (15·9 % with six or more prescriptions v. 9·7 %, P < 0·001), different ATC drug classes reimbursed (33·6 % with more than two classes per prescription v. 27·7 %, P=0·01) and most of the study drugs (P < 0·05 for antidepressants, corticosteroids, muscle relaxants and analgesics).
Before and after comparisons
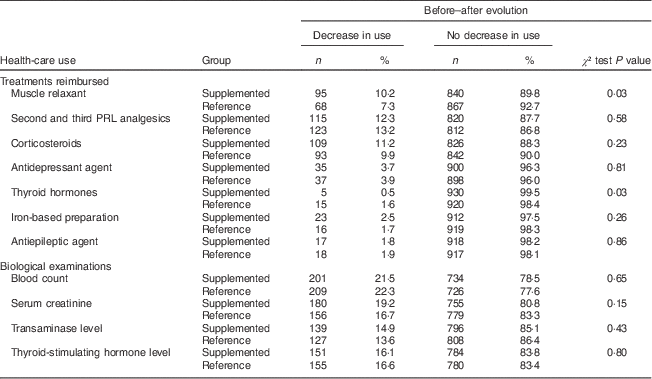
In the supplemented group, 24 % of the patients received less than 5000 µg (200 000 IU) over the 5-month follow-up, approximately 40 % of the patients received between 5000 and 10 000 µg (200 000 and 400000 IU) and 36 % received more than 10000 µg (400 000 IU). Prescriptions of drugs expected to be associated with vitamin D deficiency symptoms are shown in Table 2. In the supplemented group, fewer patients were treated by muscle relaxants after supplementation than before (13·1 % v. 8·6 %, P<0·001). Such a pattern was not observed in the reference group, where there was no change in muscle relaxant prescription frequency (10·1 % v. 10·7 %, P=0·68). Regarding second and third PRL analgesics, prescriptions tended to decrease in both groups although this did not reach statistical significance in the supplemented group (23·2 % v. 21·2 %, P=0·19 in supplemented group; 19·2 % v. 16·0 %, P=0·04 in reference group). There was no change in the number of specialists visited during the post-supplementation period compared with the pre-supplementation period in both groups. The overall number of pharmacy prescriptions per patient did not change significantly; neither did the number of sick leaves or medical imaging examinations per patient. The mean number of diagnosis-related procedures per patient did not change significantly. Between-group comparisons are displayed in Table 3. In the supplemented group, a higher proportion of patients decreased their use of muscle relaxants than in the reference group (10·2 % v. 7·3 %, P=0·03) and a lower proportion of patients decreased their use of thyroid hormones (0·5 % v. 1·6 %, P=0·03). Regarding the supplemented group, we searched for whether there was a switch from muscle relaxants towards antidepressants, but we did not observe any statistically significant relationship between decrease in use of muscle relaxants and increase in use of antidepressants. For biological tests expected to be prescribed to patients with vitamin D deficiency symptoms, the supplemented group and reference group had similar decreasing patterns.
Table 2 Frequency of prescriptions of drugs and biological examinations expected to be associated with 25(OH)D deficiency symptoms in the two groups before and after the index date; data on young to middle-aged patients who had a 25(OH)D assay between 1 December 2008 and 31 January 2009, ERASME database (regional component of the FHIF), Rhône-Alpes area, France

25(OH)D, 25-hydroxyvitamin D; ERASME, Extraction, Recherches, Analyses pour un Suivi Médico-Economique; FHIF, French Health Insurance Funds; PRL, WHO’s Pain Relief Ladder.
Table 3 Individual evolution of prescription of drugs and biological examinations expected to be associated with 25(OH)D deficiency symptoms; data on young to middle-aged patients who had a 25(OH)D assay between 1 December 2008 and 31 January 2009, ERASME database (regional component of the FHIF), Rhône-Alpes area, France

25(OH)D, 25-hydroxyvitamin D; ERASME, Extraction, Recherches, Analyses pour un Suivi Médico-Economique; FHIF, French Health Insurance Funds; PRL, WHO’s Pain Relief Ladder.
Discussion
In this population-based cohort of patients who had a 25-(OH)D assay during winter, we targeted a young to middle-aged population aged 13–60 years who was free of heavy chronic disease and assessed global health-care use as well as specific prescriptions expected to be associated with vitamin D deficiency symptoms. We observed a moderate but statistically significant decrease in some prescriptions potentially associated with vitamin D deficiency symptoms i.e. pain and asthenia, particularly in muscle relaxant prescriptions. We also observed a decrease in prescriptions of second and third PRL analgesics but this was similar to the reference group. Besides, prescription of vitamin D supplementation in patients likely to have a vitamin D deficiency was not associated with a global decrease in the other health-care consumptions as measured by the number of physician visits or the number of diagnostic or therapeutic procedures performed.
It has been described in the literature that many patients with vitamin D deficiency may suffer from unspecific chronic fatigue or musculoskeletal pain and that the mean delay from symptoms to diagnosis of vitamin D deficiency is long(Reference de Torrenté de la Jara, Pécoud and Favrat4, Reference Nellen, Smulders and Jos Frissen12–Reference Fabbriciani, Pirro and Leli15). In a before–after study conducted in Switzerland in 2005 among female asylum seekers with chronic complaints for bone pain, proximal muscular weakness, a change in gait and/or fatigue, the authors found a mean duration of symptoms of 2·5 years before a diagnosis of vitamin D deficiency was established. The mean number of emergency medical visits decreased by 44 % in the sample after the diagnosis of vitamin D deficiency and vitamin D supplementation was established. Similarly, the mean number of analgesic drugs prescribed decreased by 51 % after deficiency was treated(Reference de Torrenté de la Jara, Pécoud and Favrat4). In a previous study conducted by our university department of general practice in the Rhône-Alpes area in a cohort of 196 women with no chronic disease aged from 19 to 49 years, who wore concealing clothing and who consulted their general practitioner from January through March 2008, it was found that 95·9 % of women had a 25(OH)D level <75 nmol/l and 53·6 % a level <30 nmol/l. Of all women studied, 53 % had asthenia and 45 % had musculoskeletal pain(Reference Le Goaziou, Contardo and Dupraz3). That study was consistent with other European studies showing that all these patients with unspecific musculoskeletal pain had not been screened for 25(OH)D, and that 30–59 months had elapsed before the deficiency was diagnosed(Reference Le Goaziou, Contardo and Dupraz3, Reference McBeth, Pye and O’Neill22).
In the present study we observed a significant decrease in muscle relaxant prescriptions in patients who had a 25-(OH)D assay followed by vitamin D supplementation in the 3 months. This decrease was significant whereas no decrease was observed in the reference group. Unfortunately, we could not assess the consumption of frequently used painkillers, as most of them are delivered over the counter. That is why we had to consider only the second and third PRL analgesics. A decrease in second and third PRL analgesic prescriptions was observed in both groups and thus could not be associated with the correction of vitamin D deficiency. However, chronic pain associated with long-term vitamin D deficiency might not often require second and third PRL analgesic prescriptions but mostly first PRL analgesics.
We observed an increase in the use of thyroid hormones and antidepressants after vitamin D supplementation. This result could be explained by the fact that vitamin D deficiency investigation could be triggered by unspecific clinical signs like asthenia, which could be also due to hypothyroidism and depression. Therefore, an increase in use of pharmacotherapies indicated in hypothyroidism or depression after a 25(OH)D supplementation could signal a concomitant diagnosis of these diseases, associated with or caused by a vitamin D deficiency(Reference Mozaffari-Khosravi, Nabizade and Yassini-Ardakani23, Reference Bozkurt, Karbek and Ucan24).
We did not observe an overall decrease in health-care use after 25(OH)D supplementation in patients likely to have a vitamin D deficiency. The total number of physician visits and therapeutic and diagnostic-related procedures did not decrease. There are several possible explanations for these observations. The main limitation of our study is the lack of information regarding 25(OH)D results in our administrative database. We assumed that patients who received supplementation after a 25(OH)D assay were deficient and that those who did not were not. However, we have no information on the degree of vitamin D deficiency and it is possible that some physicians may have overprescribed vitamin D. Nevertheless, if vitamin D was prescribed in non-deficient individuals no beneficial effect on health-care use may be expected in those patients. We assumed that clinicians who prescribed 25(OH)D assays, prescribed consequently vitamin D in case of vitamin D deficiency. However, it is possible that some patients have not complied with these prescriptions. Additionally, we cannot assess the appropriateness of the dose of vitamin D prescribed and some patients may not have received sufficient doses. Another explanation for our results is the limited time frame of the study. It is possible that 5 months was not enough to observe a significant effect of the supplementation, especially if the dose was inadequate. A final explanation would be that vitamin D supplementation has a limited effect on health-care use(Reference Warner and Arnspiger25). We observed a significant decrease in muscle relaxant use and non-significant decrease in analgesic use subsequent to vitamin D supplementation, which was not associated with an increase of antidepressant use indicated in some cases of persistent neuropathic pain. There is emerging evidence on the efficacy of vitamin D to improve asthenia and/or pain in 25(OH)D-deficient patients(Reference Daniel and Pirotta26) but the efficacy of vitamin D in pain relief is still debated. A Cochrane review from 2010 stated that the existing randomized controlled trials were too small to reach meaningful conclusions regarding the hypothesis of vitamin D supplementation as a treatment for chronic pain(Reference Straube, Derry and Moore27).
Conclusion
Our findings only partially support our initial hypothesis that vitamin D supplementation of individuals with vitamin D deficiency decreases health-care use. We observed a decrease in myorelaxing drug claims only in the supplemented group v. the reference group, with no significant switch towards other musculoskeletal pain pharmacotherapies. No overall decrease in diagnostic or therapeutic medical procedures was observed. Our study highlights potential areas for future research, including the frequency of undiagnosed vitamin D deficiency associated with diffuse musculoskeletal pain in the general population and the investigation of the efficacy of vitamin D supplementation on these symptoms in randomized controlled clinical trials.
Acknowledgements
Acknowledgements: The authors wish to thank Mrs V. Ambrosi, Dr R. Nublat and Dr G. Weill (French National Healthcare System), Professor C. Colin (Department of Medical Information) and Dr M.F. Le Goaziou (Department of General Practice). Financial support: This work was funded by French public institutions, i.e. the Hospices Civils de Lyon (HCL) and the Institut National de la Santé et de la Recherche Médicale (INSERM), which had no role in the design, analysis or writing of this article. The authors had full intellectual independence regarding the funders. Conflict of interest: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that: (i) all authors have no support from companies for the submitted work; (ii) only R.C. had relationships with companies that might have an interest in the submitted work in the previous 3 years; (iii) the authors’ spouses, partners or children have no financial relationships that may be relevant to the submitted work; and (iv) all authors have no non-financial interests that may be relevant to the submitted work. Authorship: P.C. was responsible for the conception and design, analysis and interpretation of data, and drafting the paper. S.J. assisted with interpretation of data and drafting the paper and revising it critically for important intellectual content. L.L. assisted with interpretation of data and drafting the paper and revising it critically for important intellectual content. R.C. assisted with interpretation of data, logistic resources management, and drafting the paper and revising it critically for important intellectual content. M.R. assisted with analysis and interpretation of data, and drafting the paper and revising it critically for important intellectual content. M.D. assisted with interpretation of data and drafting the paper and revising it critically for important intellectual content. A.-M.S. was responsible for the conception and design, analysis and interpretation of data, and drafting the paper and revising it critically for important intellectual content. Patients’ consent to publication: Consent was not obtained but the presented data are anonymised and risk of identification is low. These data were used with approval of the National Informatic and Liberty Commission (CNIL). Access to the data: All authors had unrestricted access to the data. ENCEPP registration number: ENCEPP/SDPP/3260.