Background
Shared decision-making (SDM) has been described by Elwyn et al as ‘an approach where clinicians and patients share the best available evidence when faced with the task of making decisions, and where patients are supported to consider options to achieve informed preferences’.Reference Elwyn, Frosch, Thomson, Joseph-Williams, Lloyd and Kinnersley1 SDM has been recommended by the World Health Organization for reducing the global burden of chronic diseases,2 and is commonly considered to be the foundation of person-centred care.Reference Barry and Edgman-Levitan3,Reference Weston4
Previous research suggests that SDM may be associated with enhanced patient-reported outcomes, such as satisfaction with care, patient activation, patient knowledge gain, self-care and health-related quality of life, particularly among adults with chronic diseases such as diabetes, cardiovascular disease and dementia.Reference Rodriguez-Osorio and Dominguez-Cherit5–Reference Saheb Kashaf, McGill and Berger7 Some scholars have advocated for the use of SDM in patients with mental health disorders;Reference Drake, Cimpean and Torrey8,Reference Hamann and Heres9 however, evidence for SDM in patients with mental health conditions has been less convincing. For example, in 2018, Samalin et alReference Samalin, Genty, Boyer, Lopez-Castroman, Abbar and Llorca10 systematically reviewed the impact of SDM on various mood disorders, such as dysthymia, major depressive disorder and bipolar disorder. Fourteen randomised controlled trials (RCTs) were included, but only one study suggested that SDM, when facilitated by decision aids, may improve depressive symptoms, patient knowledge and health-related quality of life. The authors found only three studies investigating SDM among adults with depressive disorders, and studies of SDM in patients with anxiety disorders were excluded.Reference Samalin, Genty, Boyer, Lopez-Castroman, Abbar and Llorca10 In 2020, Fisher et alReference Fisher, Mills, Teesson and Marel11 conducted a systematic review evaluating the effect of SDM among patients with mental ill health and substance/alcohol use disorder comorbidities. The authors found ten studies cautiously suggesting that SDM may be ‘acceptable, feasible and beneficial’ in this population.Reference Fisher, Mills, Teesson and Marel11 A recent meta-analysis suggests that ‘patient-centred communication’ may increase therapeutic alliance.Reference Pinto, Ferreira, Oliveira, Franco, Adams and Maher12
Rationale for review
As a result, we believe that is worthwhile to explore whether SDM may be beneficial for individuals with anxiety and/or depressive disorders. SDM may be suitable in this population for various reasons. For instance, anxiety and/or depressive disorders commonly co-occur, and are often treated by using a combination of various pharmacotherapeutic and psychotherapeutic approraches.Reference Bandelow, Michaelis and Wedekind13,Reference Bschor and Adli14 Several classes of antidepressants exist, with each showing comparable safety and effectiveness.Reference Moncrieff and Kirsch15–Reference Geddes, Carney, Davies, Furukawa, Kupfer and Frank18 Additionally, several types of effective psychotherapies exist, such as cognitive–behavioural therapy, interpersonal therapy, dialectical behaviour therapy, behavioural activation therapy and problem-solving therapy, among others.Reference Cuijpers, Van Straten, Andersson and Van Oppen19 Therefore, involving the patient in decisions pertaining to treatment selection may be largely based on the patient's personal preferences, and is unlikely to have a deleterious effect on health outcomes when the treatment regime is evidence-based.Reference Stahl20
Aims
Here, we aimed to investigate whether SDM enhances clinically relevant outcomes in adults (aged 18–64 years) with anxiety and/or depressive disorders. This systematic review provides a contribution to the literature by (a) using a novel, evidence-based conceptualisation and operationalisation of SDM; and (b) assessing clinically relevant outcomes identified by an expert panel of clinicians.
Method
The protocol for this review is published in the PROSPERO International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/PROSPERO), under registration number 126079. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) StatementReference Moher, Liberati, Tetzlaff, Altman and Group21 was consulted to guide the conduct and reporting of this review. Since this was a secondary analysis of published data, ethics approval was not required.
Eligibility criteria
Table 1 displays the full eligibility criteria. The a priori inclusion/exclusion criteria was adapted from the Population, Intervention, Control, Outcome, Setting (PICOS) model.Reference Saaiq and Ashraf22
Table 1 Inclusion/exclusion criteria
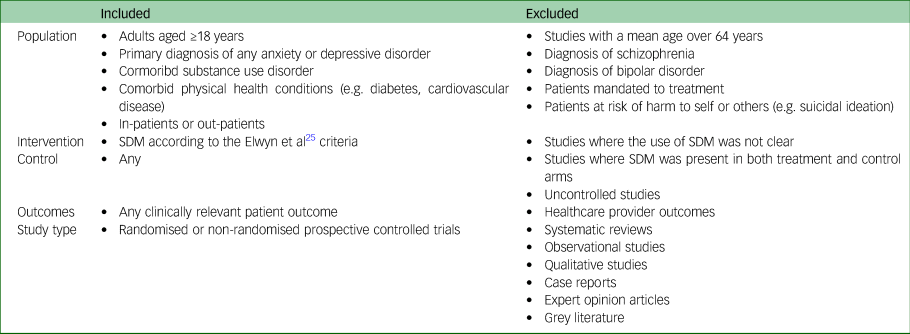
SDM, shared decision-making.
Participants
Studies of adults (aged >17 years) diagnosed with either an anxiety and/or depressive disorder were included. Studies of participants aged <18 years were excluded because a proxy decision maker is often required.Reference Lipstein, Brinkman and Britto23,Reference McCarus, Wiercinski and Heidrich24 Studies of older adults (aged >64 years) were excluded as this population was outside the scope of the review. Studies of patients with other mental and/or physical health comorbidities were included, except for severe mental illnesses such as bipolar disorder, schizophrenia or suicidality. Patients mandated to treatment, for any reason, were excluded.
Intervention
The intervention must have an equivalent to SDM as per the definition from Elwyn et al.Reference Elwyn, Laitner, Coulter, Walker, Watson and Thomson25 We used the following criteria to screen eligible studies: patients were provided options or choices regarding their healthcare; patients and healthcare providers made decisions together, informed by the best available evidence; and patients’ preferences were considered by the healthcare provider.
In cases of uncertainty, we contacted the corresponding author of the study to obtain confirmation that the intervention met our a priori criteria for SDM.
Control
Only studies with a control group (e.g. active controls, sham controls, treatment-as-usual) were included.
Outcomes
Studies must have reported patient outcomes for eligibility. For example, studies that only reported healthcare provider outcomes were excluded.
Study type
Only prospective controlled trials published in peer-reviewed journals were included.
Search strategy
A health research librarian assisted with developing and conducting the search strategy. Medline, EMBASE, PsycINFO, the Cochrane Database for Controlled Trials and the Cochrane Database for Systematic Reviews were searched from database inception until 18 August 2019. Reference lists of included texts were searched to ensure any remaining relevant articles were identified. Because of feasibility restraints, only studies published in English were considered. The search strategy used free-text and medical subject headings derived from a relevant scoping review.Reference Marshall, Kinnard, Hancock, King-Jones, Olson and Abba-Aji26 Supplementary File 1 available at https://doi.org/10.1192/bjo.2021.1028 displays the preliminary Medline search strategy.
Study selection
Endnote for Windows (Version X9, Clarivate Analytics, Philadelphia, PA, US; see http://www.endnote.com) was used to manage the references and full-text PDFs. One reviewer screened the titles and abstracts, and another reviewer screened the excluded articles to ensure relevant articles were not inadvertently discarded. Two reviewers then independently reviewed the full text of each article to assess the inclusion/exclusion criteria. A third reviewer was consulted to arbitrate any disagreements.
Data extraction
The included references were exported into a Microsoft Excel spreadsheet to complete the data extraction process. The data extraction form was developed a priori and piloted using two randomly selected studies. The data extraction form was finalized on 18 November 2019. Two reviewers independently extracted data from the remaining included studies. We contacted the corresponding authors of the included studies to request any missing data. Any discrepancies in data extraction between the reviewers were resolved by consensus.
Data items
The following data items were extracted: bibliographic information (first author, title, year of publication and country); general study characteristics (study objectives, study design, setting, duration, data collection information); participant characteristics (number of patients, number of healthcare providers, age range, mean age, gender, diagnosis); methodological characteristics (number patients allocated to intervention and control, description of intervention, description of control, description of SDM measurement, reported outcomes); and main findings (effect size, P-value, drop-out rate, adverse events, author's conclusion and limitations).
Risk of bias within studies
Two authors independently assessed the risk of bias for each study, using the Cochrane Risk of Bias Tool.Reference Higgins, Altman, Gotzsche, Juni, Moher and Oxman27 Disagreements were resolved by discussion. The results of the risk-of-bias assessment were produced in Review Manager (RevMan) for Windows (Version 5.4. The Cochrane Collaboration, Copenhagen, DEN; see http://www.training.cochrane.org). The quality of the outcome evidence was assessed by two independent reviewers, using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE)Reference Alonso-Coello, Oxman, Moberg, Brignardello-Petersen, Akl and Davoli28 tool. A third reviewer was available to arbitrate any disagreements.
Risk of bias across studies
An assessment of publication bias was planned with a funnel plot, where feasible.
Synthesis of results
A meta-analysis was planned if a sufficient number of clinically homogenous articles were retrieved. Alternatively, a narrative synthesis was planned if meta-analysis was not possible.
Additional analyses
Three subgroup analyses were conducted. First, a subgroup analysis of studies of emerging adults (individuals aged 18–25 years) was conducted a priori. Corresponding authors of the included studies were contacted to request data on this age group when possible. This age group was selected because there is reason to suspect that emerging adults may be more receptive to SDM compared with older adults.Reference Sussman and Arnett29,Reference Monaghan30 For example, emerging adults have shown to be more likely to prefer autonomy in health decision-making and self-management of mental health symptoms.Reference Samalin, Genty, Boyer, Lopez-Castroman, Abbar and Llorca10,Reference Duncan, Best and Hagen31 Moreover, anxiety and depressive disorders are widespread globally, and are becoming increasingly common in this age group,Reference Patel, Flisher, Hetrick and McGorry32 but seldom receive timely and adequate care.Reference Patel, Flisher, Hetrick and McGorry32,Reference Spence, Owens-Solari and Goodyer33
Second, we conducted a post hoc analysis comparing studies of collaborative care involving SDM with studies with SDM interventions without collaborative care. Collaborative care is considered a patient-centred approach, involving the use of a multidisciplinary behavioural healthcare team, typically led by a primary healthcare provider.Reference Shaw, Sethi, Vaccaro, Beatty, Kirsten and Kissane34–Reference Archer, Bower, Gilbody, Lovell, Richards and Gask36 Collaborative care typically recommends incorporating patient goals into the treatment plan, but may not explicitly recommend or measure SDM.Reference Goodrich, Kilbourne, Nord and Bauer37
Third, studies of SDM facilitated by decision aids were analysed separately in a post hoc analysis. This analysis may be useful because it is unclear whether decision aids enhance the effects of SDM. Some research suggests that SDM does not require the use of a physical decision aid to be effective.Reference Tse38 Other studies have suggested that decision aids may promote SDM,Reference Ubbink, Knops, Molenaar and Goossens39 and decision aids have been recommended by Elwyn et alReference Elwyn, Frosch, Thomson, Joseph-Williams, Lloyd and Kinnersley1 to facilitate evidence-based decision-making when engaging in SDM.
Results
Study selection
Figure 1 illustrates the study selection process. The search of electronic health databases initially retrieved 10 621 publications. After discarding duplicates, we obtained 7424 references, which were screened by title and abstract. Eighty articles were retained for screening in full text. As part of this evaluation, we contacted the corresponding authors of 19 studies to verify whether the trialled intervention met our a priori definition for SDM. Three additional studiesReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40–Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 were included as a result. Supplementary File 2 displays the results of this inquiry. Six studies were included for the final data synthesis.
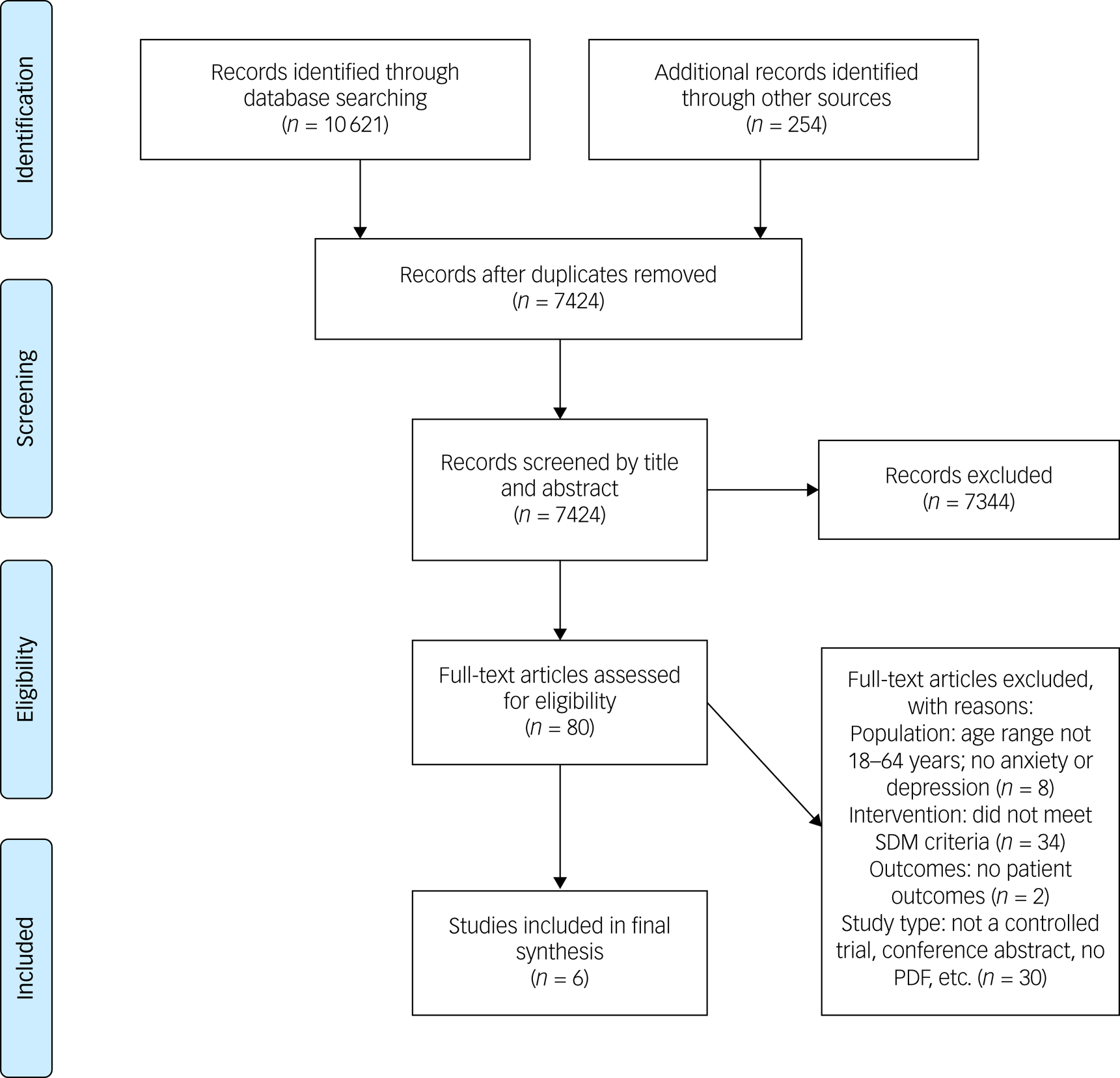
Fig. 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart. SDM, shared decision-making.
Characteristics of included studies
Table 2 displays the characteristics of included studies. Six RCTsReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40–Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 met the inclusion criteria (N = 1834 participants). The publication dates ranged from 2007 to 2015. All six studies included patients who were diagnosed with a depressive disorder; only two studies included patientsReference Grote, Katon, Russo, Lohr, Curran and Galvin41,Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 with anxiety disorders. Four of the six studiesReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40–Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42,Reference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44 were conducted in the USA, one in GermanyReference Loh, Simon, Wills, Kriston, Niebling and Harter45 and one in Saudi Arabia.Reference Aljumah and Hassali43 Three of the six studies were parallel RCTsReference Grote, Katon, Russo, Lohr, Curran and Galvin41–Reference Aljumah and Hassali43 and three were cluster RCTsReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40,Reference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44,Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 in which physician clinics, rather than participants, were randomised to the intervention group. Two studies were conducted in an in-patient hospital setting,Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42,Reference Aljumah and Hassali43 three studies were conducted in primary care settingsReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40,Reference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44,Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 and one study was conducted at a public health centre.Reference Grote, Katon, Russo, Lohr, Curran and Galvin41 The patient population varied considerably. Three studiesReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40,Reference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44,Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 involved adults (aged 18–64 years) with depression in primary care. One studyReference Grote, Katon, Russo, Lohr, Curran and Galvin41 included only women with perinatal depression and low socioeconomic status. Another studyReference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 included only in-patients with moderate-to-severe depression in addition to comorbid cardiovascular disease.
Table 2 Characteristics of included studies

RCT, randomised controlled trial; MMAS, Morisky Medication Adherence Scale; BMQ, Patients’ Beliefs about Medicine Questionnaire (specific and general versions); MADRS, Montgomery–Åsberg Depression Rating Scale; OPTION, Observing Patient Involvement in Decision-Making Scale; TSQM 1.4, Treatment Satisfaction Questionnaire for Medication; PHQ, Patient Health Questionnaire; EBQI, Evidence-Based Quality Improvement; SF-12, Medical Outcomes Study Short Form; PTSD, post-traumatic stress disorder; GAD, Generalized Anxiety Disorder; SCL-20, Hopkins Symptom Checklist; WSAS, Work and Social Adjustment Scale; PCL-C, Post-Traumatic Stress Disorder Checklist-Civilian Version; DCS, Decisional Conflict Scale; PICS, Patient's Perceived Involvement in Care Scale; MSH, Man-Son-Hing instrument; PHQ-D, Patient Health Questionnaire-Depression; CSQ-8, Client Satisfaction Questionnaire.
Three of the six studiesReference Aljumah and Hassali43–Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 employed interventions where SDM was explicitly defined a priori. Two of theseReference Aljumah and Hassali43,Reference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44 quantitatively measured the SDM with a validated instrument such as the Observing Patient Involvement in Decision-Making Scale (OPTION) tool,Reference Elwyn, Edwards, Wensing, Hood, Atwell and Grol46 and one studyReference Loh, Simon, Wills, Kriston, Niebling and Harter45 measured SDM by using a combination of the Patient Perceived Involvement in Care ScaleReference Lerman, Brody, Caputo, Smith, Lazaro and Wolfson47 and the patient participation scale (Man-Son-Hing instrument). One studyReference Aljumah and Hassali43 assessed a pharmacist intervention based on SDM that provided direct patient care compared with usual pharmacy services. The other two studies that explicitly defined SDMReference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44,Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 involved the use of an evidence-based decision aid for antidepressants, compared with primary care as usual with no decision aid. Three studiesReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40–Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 consisted of collaborative care interventions in which SDM was confirmed to be incorporated into the intervention by the corresponding authors. Collaborative care was generally delivered as a multicomponent depression intervention, including patient education and treatment coordination led by a patient care manager.Reference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40-Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42
Risk of bias within studies
Figure 2 displays the results of the risk-of-bias assessment. The included RCTs were at moderate risk of bias. Two studies did not report random sequence generation methods;Reference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40,Reference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44 five studies did not report allocation concealment strategies;Reference Fisher, Mills, Teesson and Marel11,Reference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40,Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42–Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 two studiesReference Aljumah and Hassali43,Reference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44 did not blind the participants or outcome assessors, and three studies did not provide a clear description;Reference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40,Reference Grote, Katon, Russo, Lohr, Curran and Galvin41,Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 one studyReference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44 did not adequately blind the outcome assessor, three studies were unclear;Reference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40,Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42,Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 two studiesReference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44,Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 suffered from incomplete reporting of outcome data, and one study did not clearly pre-specify outcomes.Reference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40 No other sources of bias were identified.

Fig. 2 Risk-of-bias (ROB) summary of results. Legend: green, low ROB; red, high ROB; yellow, unclear ROB.Reference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40–Reference Loh, Simon, Wills, Kriston, Niebling and Harter45
Risk of bias across studies
Assessment of publication bias was not conducted because of an insufficient number of included studies (fewer than ten) per Cochrane recommendations.Reference Higgins, Thomas, Chandler, Cumpston, Li and Page48
Impact of SDM on clinically relevant outcomes
Table 3 displays the summary of findings according to patient outcome.
Table 3 Summary of main findings according to patient outcome

GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
Symptoms of anxiety
Only one of the six included studiesReference Grote, Katon, Russo, Lohr, Curran and Galvin41 reported whether SDM affected symptoms of anxiety. Grote et alReference Grote, Katon, Russo, Lohr, Curran and Galvin41 found that a collaborative care intervention inclusive of SDM (MOMCare and Maternity Support Services-Plus (MSS-Plus), n = 83 participants) reduced the number of participants who met the criteria for anxiety on the Generalized Anxiety Disorder-7 questionnaire compared with the control condition (MSS-Plus, n = 81 participants) at the 18-month follow-up (n = 152; intervention 10.0% v. control 22.2%; odds ratio 0.39; 95% CI 0.16−0.97). Although there was a medium effect size of 0.52, only one study reported this outcome, resulting in low certainty of evidence owing to imprecision in using the GRADE criteria.Reference Guyatt, Oxman, Kunz, Brozek, Alonso-Coello and Rind49
Symptoms of depression
Five of the six included studiesReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40,Reference Grote, Katon, Russo, Lohr, Curran and Galvin41,Reference Aljumah and Hassali43–Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 reported whether SDM affected symptoms of depression. Grote et alReference Grote, Katon, Russo, Lohr, Curran and Galvin41 reported that a collaborative care intervention inclusive of SDM (MOMCare) was associated with a decrease in mean depression severity on the 20-item Hopkins Symptom Checklist (SCL-20) compared with the control (MSS-Plus) at the 6-month follow-up (n = 157; mean difference −0.24; 95% CI −0.46 to 0.03; P = 0.03) and 18-month follow-up (n = 152; mean difference −0.25; 95% CI −0.45 to 0.04; P = 0.02). Four studiesReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40,Reference Aljumah and Hassali43–Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 reported no change in depression severity compared with control conditions. Depression was measured in a variety of ways, including depression severity, using the Patient Health Questionnaire-9, SCL-20 and Hospital Anxiety and Depression Scale. Given the variation in outcome results, the certainty of the evidence was rated moderate because of inconsistency.Reference Guyatt, Oxman, Kunz, Brozek, Alonso-Coello and Rind49
Health-related quality of life
One of the six studiesReference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42,Reference Aljumah and Hassali43 reported whether or not SDM affected health-related quality of life. Aljumah et alReference Aljumah and Hassali43 reported no change in health-related quality of life resulting from usual pharmacy services plus pharmacist interventions based on SDM compared with usual pharmacy services after 6 months of follow-up. Health-related quality of life was measured with the EuroQol-5D. Given that only one included study was available for the GRADE rating and the study results were inconclusive, the certainty of the evidence on health-related quality-of-life outcomes was rated low because of imprecision.Reference Goodrich, Kilbourne, Nord and Bauer37
Likelihood of receiving evidence-based depression treatment
Three research teamsReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40–Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 reported whether SDM affected the probability of receiving adequate depression treatment. Adequate depression treatment was defined by Huffman et alReference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 as either prescription of an antidepressant at a clinically effective dose according to treatment guidelines, or referral to a mental health treatment provider for psychological therapy. This was measured by the medication possession ratioReference Kozma, Dickson, Phillips and Meletiche50 and by obtaining information from patient charts.
The researchersReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40–Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 also found that the collaborative care models involving SDM increased the likelihood of receiving either antidepressant therapy or referral to psychological therapy. Chaney et alReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40 found that the evidence-based quality improvement collaborative care model (EBQI-CCM) intervention (n = 268) improved the likelihood of receiving an adequate dosage of antidepressant therapy (65.7% received adequate dosage) at 7 months after baseline compared with the non-EBQI-CCM intervention (n = 238, 43.4% received adequate dosage; difference 22.3, P < 0.001). Grote et alReference Grote, Katon, Russo, Lohr, Curran and Galvin41 reported that the MOMCare and MSS-Plus (n = 83) intervention group had higher rates of antidepressant use regarding group (χ² = 8.10, d.f. = 1, P < 0.01) and time (χ² = 18.67, d.f. = 3, P < 0.0001) effects, compared with the MSS-Plus control group (n = 81). The intervention group also displayed a higher adherence rate than the control group, with statistical significance (χ² = 10.00, d.f. = 1, P = 0.002). In addition, Huffman et alReference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 found that the collaborative care intervention group (n = 90) had a significantly higher likelihood of being prescribed adequate depression treatment compared with the usual care control group (n = 85) (intervention: 64 out of 89 (71.9%) v. control: 8 out of 84 (9.5%); χ² = 71.46; d.f. = 1; P < 0.001). The effect size was 0.66 at 3 months, 0.52 at 6 months, 0.54 at 12 months and 0.05 at 18 months. The certainty of the evidence was downgraded to moderate because of risk of bias among the included studies.
Patient satisfaction with care
The research teams of five of the six studiesReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40,Reference Grote, Katon, Russo, Lohr, Curran and Galvin41,Reference Aljumah and Hassali43–Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 discussed whether SDM affected patient satisfaction with care. Four groupsReference Grote, Katon, Russo, Lohr, Curran and Galvin41,Reference Aljumah and Hassali43–Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 found an increase in patient satisfaction as a result of SDM interventions, whereas one groupReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40 found no statistically significant difference. Aljumah et alReference Aljumah and Hassali43 indicated that after 3 months of treatment, the group that received pharmacy services based on SDM (n = 110) had significantly higher scores on treatment satisfaction than the group that received usual pharmacy services (n = 110; t = 2.326, P = 0.021). After 6 months of treatment, the effect on treatment satisfaction was still statistically significant (t = 3.551, P < 0.0001). Grote et alReference Grote, Katon, Russo, Lohr, Curran and Galvin41 found that the MOMCare group participants (n = 83) reported higher mean levels of satisfaction compared with the MSS-Plus group (n = 81) at follow-up, with no significance in the main effect for time (χ² = 0.36, d.f. = 3, P = 0.95), but statistical significance for group main effect (χ² = 8.28, d.f. = 1, P = 0.004).
LeBlanc et alReference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44 reported patients in the decision aid group (n = 158) expressed higher overall satisfaction with treatment compared with the control group (n = 139) (relative risk ranging from 1.25 (P = 0.81) to 2.40 (P = 0.002)). There was no significant effect found between groups on the whether the ‘right amount of information was given’ (P = 0.81) or ‘information given was extremely clear’ (P = 0.09). Loh et alReference Loh, Simon, Wills, Kriston, Niebling and Harter45 found that patients in the patient-centred decision aid intervention group (n = 128) had significantly higher satisfaction levels at the post-intervention stage compared with the control group (n = 66; P = 0.014). However, since this questionnaire measurement tool was not administered at baseline, no temporal comparison of differences in satisfaction was possible. Chaney et alReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40 found no statistical significance for overall patient satisfaction; the author stated that 62.4% of the EBQI-CCM intervention group (n = 288) reported being ‘satisfied or very satisfied with mental healthcare’. Moreover, 67.3% of the non EBQI-CCM group (n = 258) answered the survey question affirmatively (P = 0.27).
Treatment adherence
Three of the six research teamsReference Aljumah and Hassali43–Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 studied the influence of SDM on treatment adherence. In one case,Reference Aljumah and Hassali43 the intervention was a provided by a pharmacist and reported a significant difference between the intervention and the control group. The authors of the other two studies reported no significant difference between treatment groups. Aljumah et alReference Aljumah and Hassali43 found that at the 3-month follow-up, the intervention group (n = 110) reported significantly higher scores for medication adherence compared with the control group (n = 110; t = 2.88, P = 0.004). Statistically significant results were also observed at the 6-month follow-up (t = 4.0598, P < 0.001). LeBlanc et alReference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44 reported that no clinical variation was found between treatment adherence percentages for patients in the decision aid arm (n = 158, 86.2%) and the control arm (n = 135, 93.2%) (P = 0.19). Loh et alReference Loh, Simon, Wills, Kriston, Niebling and Harter45 found similar results, where no statistical differences in patient-reported adherence were found between the patient-centred intervention group (n = 191) and the control condition participants (n = 96; P = 0.73). This was also the case for physician-rated treatment adherence in the intervention and control groups (P = 0.56). Because of the inconsistency in outcome definitions and measurements across the articles, the certainty of the evidence was low.
Patient involvement in health decision-making
Three of the six research studiesReference Grote, Katon, Russo, Lohr, Curran and Galvin41,Reference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44,Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 examined whether SDM affected patient involvement or engagement in health decision-making. Grote et alReference Grote, Katon, Russo, Lohr, Curran and Galvin41 found that 97.5% of the participants in MOMCare intervention (n = 83) and MSS-Plus group (SDM) were engaged in treatment compared with 35.2% of the MSS-Plus group (n = 81; odds ratio 72.7, 95% CI 16.5−321). LeBlanc et alReference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44 used cluster-adjusted t-tests and found that the decision aid group (n = 158) was significantly more involved in the decision-making, with 47% of the intervention group participants reporting involvement compared with 33% of the control group (n = 139; P = 0.001). The certainty of the evidence was low because of the inconsistency in the outcome measurement and reporting, and low number of included studies.
Consultation time
Two of the six research teamsReference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44,Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 studied whether SDM affected consultation time. Both studies reported no change in consultation time because of using SDM facilitated by decision aids. LeBlanc et alReference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44 found no clinically significant differences in clinical encounter duration between the decision aid intervention group (n = 158) and the control group (n = 139). The mean time was 44 (s.d. = 22) minutes for the intervention group, and 48 (s.d. = 27) minutes for the control group (P = 0.47). In addition, Loh et alReference Loh, Simon, Wills, Kriston, Niebling and Harter45 reported that there was no statistical difference in clinical consultation time between the intervention (n = 191) and control groups (n = 96) for both within-group pre- and post-treatment comparisons (intervention: P = 0.48; control: P = 0.64), and between the treatment arms (P = 0.68). The quality of the evidence obtained was low because of inconsistency in reporting outcome data, and low number of included studies.
Additional analyses
Emerging adults
No studies or data were obtained involving emerging adults.
SDM versus SDM and collaborative care
Three RCTsReference Aljumah and Hassali43–Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 conducted investigations of SDM interventions without the use of collaborative care, and three RCTsReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40–Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 conducted investigations of collaborative care interventions involving SDM. The studies involving collaborative care differed by using multiple healthcare providers, coordinated by a care manager to evaluate and coordinate the personalised care of individuals with depression based upon unique needs.Reference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40–Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 Studies of SDM aloneReference Aljumah and Hassali43–Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 primarily involved interactions between one healthcare provider and the patient.
All three RCTsReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40–Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 using collaborative care models found that patients with depression were more likely to receive adequate depression treatment compared with usual care without SDM, with statistical significance (Chaney et alReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40: P = 0.001; Grote et alReference Grote, Katon, Russo, Lohr, Curran and Galvin41: P = 0.01; Huffman et alReference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42: P < 0.001); however, the studies of SDM without collaborative care did not report this outcome. All three research groups who studied SDMReference Aljumah and Hassali43–Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 found that SDM improved satisfaction with care, and one of the two groups who studied collaborative careReference Grote, Katon, Russo, Lohr, Curran and Galvin41 reported that SDM improved satisfaction with care. Comparisons of other clinically relevant outcomes could not be conducted because of inconsistency in reporting.
SDM facilitated with a decision aid
Two research teams used decision aids to facilitate SDM compared with treatment as usual.Reference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44,Reference Loh, Simon, Wills, Kriston, Niebling and Harter45 No change was found in depressive symptoms or treatment adherence in either study. One research team who studied decision aids reported an increase in patient knowledge;Reference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44 both research groups who studied SDM and decision aids reported improvements in satisfaction with care. However, two of the three teams who studied SDM without decision aidsReference Grote, Katon, Russo, Lohr, Curran and Galvin41,Reference Aljumah and Hassali43 also reported improved satisfaction with care. Both research teams who studied SDM with decision aids found that patient participation improved, but the only team studying SDM without decision aidsReference Grote, Katon, Russo, Lohr, Curran and Galvin41 also found that patient participation improved because of collaborative care. Both teams that studied SDM studies with decision aids reported no change in consultation time. Comparisons with studies without decision aids were not possible because of limitations in outcome reporting.
Discussion
To our knowledge, this is the first systematic review to examine whether SDM affects clinically relevant outcomes in adults diagnosed with anxiety and/or depressive disorders. We found preliminary evidence suggesting that SDM may favourably affect outcomes such as satisfaction with care and patient involvement in decision-making. We found evidence that SDM, when used in conjunction with a collaborative care intervention, likely improves the likelihood of receiving adequate depression treatment, and preliminary evidence that SDM may facilitate a reduction in symptoms of anxiety. However, only one study reported anxiety as an outcome, and a statistically significant difference was only detected at 18 months of follow-up.Reference Grote, Katon, Russo, Lohr, Curran and Galvin41 It appears unlikely that SDM by itself improves symptoms of depression, as only one of the five studies that measured this outcome demonstrated a statistically significant improvement. The lone studyReference Grote, Katon, Russo, Lohr, Curran and Galvin41 that demonstrated an improvement also used collaborative care in addition to SDM, which may be partially responsible for the observed effect.
Among the included studies, only one study suggested that collaborative care involving SDM may improve symptoms of anxiety or depression.Reference Grote, Katon, Russo, Lohr, Curran and Galvin41 SDM and collaborative care studies appeared to show promising results on improving outcomes such as patient satisfaction with care, but the SDM and collaborative care studies largely measured different outcomes. Over the past 20 years, a considerable evidence base has grown in support of collaborative care for improving outcomes in patients with anxiety and depression. For example, several large systematic reviews and meta-analyses have shown that collaborative care is more effective than usual care in improving both quality-of-care outcomes and symptoms of anxiety and depression in both the short and long term.Reference Gilbody, Bower, Fletcher, Richards and Sutton35,Reference Archer, Bower, Gilbody, Lovell, Richards and Gask36,Reference Li, Kennedy, Byrne, Gerin-Lajoie, Katz and Keshavarz51 Therefore, we hypothesise that it is possible that collaborative care may either facilitate or enhance SDM.
Two included studiesReference Elwyn, Laitner, Coulter, Walker, Watson and Thomson25,Reference Harter, Buchholz, Nicolai, Reuter, Komarahadi and Kriston52 suggested that decision aids may facilitate SDM, but it was unclear from our data whether decision aids affected the effectiveness of SDM in our target population. We were only able to compare the effects of SDM facilitated via a decision aid on two outcomes (satisfaction with care and patient participation), and both approaches were beneficial. More research is needed to clarify the effect of decision aids on the effectiveness of SDM in people with anxiety and/or depressive disorders. Additionally, no relevant studies were found in the emerging adult population, and the corresponding authors of the included studies were not able to provide any additional data to conduct any further analysis. More research is needed to explore the potential effects of SDM in emerging adults, as there is evidence that emerging adults may avoid mental healthcare services and may benefit from approaches that facilitate increased engagement.Reference Spence, Owens-Solari and Goodyer33,Reference Burns and Birrell53–Reference Betz, Lobo, Nehring and Bui57
The findings of our systematic review are largely consistent with recent systematic reviews of SDM in the treatment of other mental health disorders.Reference Samalin, Genty, Boyer, Lopez-Castroman, Abbar and Llorca10,Reference Fisher, Mills, Teesson and Marel11,Reference Duncan, Best and Hagen31 Samalin et alReference Samalin, Genty, Boyer, Lopez-Castroman, Abbar and Llorca10 found 14 RCTs that used SDM or collaborative care with SDM among patients with bipolar or depressive disorders. The authors suggested improvements in patient satisfaction and engagement in decision-making were consistent across studies. Fisher et alReference Fisher, Mills, Teesson and Marel11 found ten studies of SDM in adults with co-occurring substance use and mental health disorders. This systematic review suggested that SDM was likely to be acceptable and feasible for patients with these conditions; more research was recommended by the authors.Reference Fisher, Mills, Teesson and Marel11 Neither systematic review specifically evaluated the effects of SDM in people with anxiety disorders or in emerging adults.
We aimed to expand upon this work by searching for SDM studies of anxiety disorders and conducting subgroup analyses around the effects of SDM in emerging adults, and by separately analysing studies aiming to facilitate SDM via a decision aid.Reference Samalin, Genty, Boyer, Lopez-Castroman, Abbar and Llorca10 In keeping with previous systematic reviews, we also found evidence that SDM primarily improved outcomes such as satisfaction with care and patient involvement in decision-making.Reference Samalin, Genty, Boyer, Lopez-Castroman, Abbar and Llorca10,Reference Fisher, Mills, Teesson and Marel11,Reference Duncan, Best and Hagen31 Our review, however, highlights a substantial gap in the literature around SDM in mental healthcare, as only two studiesReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40,Reference Grote, Katon, Russo, Lohr, Curran and Galvin41 included adults with anxiety disorders, and only one studyReference Grote, Katon, Russo, Lohr, Curran and Galvin41 measured symptoms of anxiety, which is insufficient for drawing firm conclusions. Future research in this area is warranted, as the studyReference Grote, Katon, Russo, Lohr, Curran and Galvin41 suggested that collaborative care intervention (with author-confirmed use of SDM) was associated with a decrease in anxiety and post-traumatic stress disorder symptom severity among socioeconomically disadvantaged women with perinatal depression.
As a result, we hypothesise that SDM may not directly ameliorate symptoms of anxiety or depression; rather, the effects of SDM may be mediated via improvements around patient-valued and/or -reported outcomes, such as therapeutic alliance, satisfaction with care, patient healthcare engagement and patient knowledge gain. Improvements in patient-reported outcomes may affect additional outcomes over time, such increased probability of selecting of appropriate treatments or medications, and adherence to these treatments, which in turn, may result in improved mental health outcomes.Reference Geddes, Carney, Davies, Furukawa, Kupfer and Frank18
SDM may be particularly beneficial during deliberation and selection of various effective treatment options (e.g. pharmacotherapy and/or psychotherapy), or during selection of specific medications and/or psychotherapies.Reference McCarus, Wiercinski and Heidrich24,Reference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44,Reference Higgins, Thomas, Chandler, Cumpston, Li and Page48 Therapeutic alliance and treatment adherence may improve when SDM is practiced consistently during each clinical encounter.Reference LeBlanc, Herrin, Williams, Inselman, Branda and Shah44 A majority of included studies measured/assessed SDM during a single clinical encounter, thus precluding any ability to observe additional benefit SDM may provide when practiced longitudinally. As a result, we hypothesise that continued engagement with the patient in a person-centred approach over time may be more beneficial than a single engagement.Reference Hamann and Heres9,Reference Tlach, Wusten, Daubmann, Liebherz, Harter and Dirmaier58,Reference Gergel, Szmukler, Molodynski, Rugkåsa and Burns59
Conversely, SDM may not be applicable in every clinical circumstance, particularly if there is an emergency situation and the patient is at risk of harm to self or others; the patient's illness significantly impairs decision-making capacity, clearly impeding the ability to make sound choices; or the patient prefers to defer decision-making to a healthcare provider.
In light of these circumstances, SDM may also be attempted with a proxy decision maker (e.g. family member or caregiver), if required. Future research is warranted to explore the effect of SDM when practiced frequently/consistently, clarify when SDM may be impossible/inappropriate, and identify ways to adapt/modify SDM during challenges clinical situations.
Limitations of the included studies
The included studies were at moderate risk of bias, which weakened the certainty of the evidence. The primary issues were around concealment of allocation and inconsistently defined and measured SDM interventions. Moreover, to the best of our knowledge, there is no universally accepted definition of SDM in the literature. The lack of a consistently defined construct of SDM in the literature poses challenges for researchers to assess the occurrence and effectiveness of SDM across multiple studies.Reference LeLaurin KW60,Reference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40–Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 Additionally, the reporting of SDM remained unclear in several potentially eligible studies, posing challenges for the review. We recommend clear reporting of SDM and how it was measured in any future studies, regardless of whether SDM is the primary intervention.
Only two of the six studies measured SDM using a validated instrument such as the OPTION scale,Reference Melbourne, Sinclair, Durand, Legare and Elwyn61 therefore it was difficult to assess to what extent SDM was delivered, and whether or not SDM was present in the control groups. Although the three collaborative care studies involving SDMReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40–Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 measured adequate depression treatment (i.e. prescribed an antidepressant based on clinical practice guidelines or referred to psychological therapy) as a primary outcome, none of the other SDM studies measured this outcome. Our observation is supported by a recent scoping reviewReference Siyam, Shahid, Perram, Zuna, Haque and Archundia-Herrera62 that also found inconsistencies among SDM outcomes and tools, which may ultimately reflect heterogeneity in how SDM is defined and measured. Validated and consistent outcome reporting in future clinical trials on SDM is needed to further synthesise the results required to better inform policy and clinical practice.
Strengths and limitations of the review
Our study has several strengths. We performed a comprehensive search spanning five healthcare databases, using literature-informed search terms related to SDM, anxiety and depressive disorders. A unique aspect of this study was that we operationally defined SDM and created rigorous inclusion criteria based on this definition. Furthermore, we contacted authors of studies where it was unclear whether SDM occurred. Two independent reviewers performed all stages of screening and analysis. Additionally, this systematic review included several clinically relevant outcomes that may be useful for informing clinical practice and policy.
There are also several limitations of this review that may affect the interpretation of findings. SDM is sometimes studied in other study types such as observational or qualitative studies, and the exclusion of these study types may not provide a clear map of all evidence investigating SDM in adults with anxiety and depression. However, including studies with these methods would have increased methodological heterogeneity in the review, and would not have helped address our review objectives. Because of feasibility concerns, we did not search for literature outside of the English language, which may have limited the inclusion of potentially relevant studies. This limitation may weaken the generalisability of this review. Since the objective of this review was SDM, we did not design a search strategy around collaborative care. We acknowledge that collaborative care may sometimes involve SDM, and therefore these studies may have been missed in our search.
Clinical heterogeneity across the included studies posed challenges for data synthesis and the interpretation of the results. For example, three of the studies involved patients with depression from primary care settings, whereas the other three studies involved specific populations such as in-patients with cardiovascular disease, women with perinatal depression and psychiatric in-patients receiving care from pharmacists. Moreover, each of these studies delivered SDM in a unique way, which made making comparisons difficult and combining results via meta-analysis not possible. For example, three of the six studiesReference Chaney, Rubenstein, Liu, Yano, Bolkan and Lee40–Reference Huffman, Mastromauro, Sowden, Wittmann, Rodman and Januzzi42 used a collaborative care intervention in addition to SDM. Notably, the collaborative care approach in these studies involved multiple modalities in addition to SDM, which may have increased the likelihood of observing a significant effect. Furthermore, meta-analysis was also not possible because of inconsistency in outcome reporting and heterogeneity in both the measurement of the independent variable (e.g. SDM) and the outcome measurements. Additionally, because of the low number of included studies, we were not able to assess publication bias, which cannot be entirely dismissed.
Overall, the obtained evidence cautiously suggests SDM may have more benefits than risks in the treatment of adults with anxiety and/or depressive disorders. The low number of studies and high clinical heterogeneity among the included studies precludes drawing firm conclusions; however, our findings are consistent with previous systematic reviews on SDM in patients with other chronic conditions. More high-quality research that uses a consistent definition (and reliable measurements) of SDM is needed to advance knowledge in the field. Additional research around SDM in emerging adults with mental health concerns, and adults with anxiety disorders, is also recommended.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjo.2021.1028
Data availability
The data that support the findings of this study are available from the corresponding author, S.V., upon reasonable request.
Acknowledgements
We would like to thank Dr. Sussane King-Jones and Myles Hancock for their assistance with this research.
Author contributions
All authors have made significant intellectual contribution to the development of this manuscript. T.M. and S.V. developed the research questions and methods. T.M. produced the first draft of the manuscript. C.S., K.O., A.A.-A., R.L. and X.-M.L. assisted in refinement of the research questions and revisions of the manuscript. T.M. and a health research librarian with systematic review expertise designed the search strategy and performed the database searches. T.M. and C.S. screened, extracted and analysed the data. S.V. has expertise in systematic reviews and provided detailed feedback on all manuscript drafts. All authors have contributed substantially to the study conception, acquisition, analysis and interpretation of data, and have approved the final version of this manuscript.
Funding
This research has been funded by the Stollery Children's Hospital Foundation through the Women and Children's Research Institute via a grant awarded to T.M.. No grant number was provided for this particular funding.
Declaration of interest
None.









eLetters
No eLetters have been published for this article.