Article contents
Use of ultrasound with magnetic field for enhanced in vitro drug delivery in colon cancer treatment
Published online by Cambridge University Press: 28 March 2018
Abstract
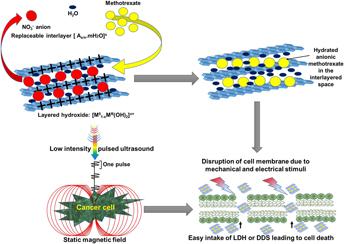
Drug delivery systems (DDSs) have been developed to target tumor cells by releasing active biomolecules at the specific site of infection, thus eliminating the side effects of anticancer drugs. However, DDSs are generally limited by high drug dosage, biobarriers, poor target recognition, etc. To address these deficiencies, we propose a new noninvasive method consisting of exposing the cancer cells to a combination of low-intensity pulsed ultrasound (LIPUS) and static magnetic field (SMF). This combined treatment found to negatively regulate colon cancer cell (HCT116) activities in vitro by altering their cell membrane potential and permeability thus increased the DDS efficacy by 40%. The treated cancer cell membrane became hyperpolarized leading to cancer cell death. The combination treatment (LIPUS + SMF) restricted the cancer cell proliferation to 16 and 5% in the presence of bare anticancer drug and DDS, respectively, in 72 h, which is almost 40% higher than that observed without the treatment. The acceleration of cancer cellular inhibition was confirmed by the significant increase in the apoptosis of the cell exposed to the LIPUS + SMF treatment. The observed improvement is believed to be due to changes in the cell membrane stability/permeability as a result of mechanical (20–22 kPa) and electrical (19–23 µV/cm) stimuli generated during the LIPUS + SMF treatment.
Keywords
Information
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2018
References
REFERENCES
- 5
- Cited by

