Book contents
- Frontmatter
- Contents
- Foreword
- Preface
- Abbreviations and Acronyms
- PART I
- PART II Heterocyclic Reactions
- 1 Algar-Flynn-Oyamada Reaction
- 2 Allan-Robinson
- 3 Auwers Synthesis
- 4 Baeyer-Drewson Indoxyl Synthesis
- 5 Bernthsen Acridine Synthesis
- 6 Betti Reaction
- 7 Biginelli Reaction
- 8 Bischler-Mohlau Indole Synthesis
- 9 Bischler-Napieralski Reaction
- 10 Borsche-Drechsel Reaction
- 11 Bucherer-Bergs Reaction
- 12 Bucherer Carbazole Synthesis
- 13 Camps Quinoline Synthesis
- 14 Chichibabin Reaction
- 15 Claisen Isoxazole Synthesis
- 16 Combes Quinoline Synthesis
- 17 Conrad-Limpach Reaction
- 18 Darzens Glycidic Ester Condensation
- 19 Doebner Reaction
- 20 Doebner-von Miller Synthesis
- 21 Erlenmeyer-Plochl Azlactone Synthesis
- 22 Fischer-Indole Synthesis
- 23 Friedlander Synthesis
- 24 Gabriel-Colman Rearrangement
- 25 Gould-Jacobs Reaction
- 26 Graebe-Ullmann Synthesis
- 27 Hantzsch Pyridine Synthesis
- 28 Hantzsch Pyrrole Synthesis
- 29 Hantzsch Thiazole, Pyridine and 1,2,4-Triazine Synthesis
- 30 Hansch Reaction (Synthesis of Benzofurans)
- 31 Hofmann-Löffler-Freytag Reaction
- 32 Knorr Pyrazole Synthesis
- 33 Knorr Pyrrole Synthesis
- 34 Knorr Quinoline Synthesis
- 35 Kostanecki-Robinson Reaction
- 36 Madelung Synthesis
- 37 Nenitzescu Indole Synthesis
- 38 Nimentowski Quinazoline Synthesis
- 39 Nimentowski Quinoline Synthesis
- 40 Paal-Knorr Synthesis
- 41 Paterno-Buchi Reaction
- 42 Payne Rearrangement
- 43 Pechmann Condensation
- 44 Pfitzinger Quinoline Synthesis
- 45 Pictet-Gams Isoquinoline Synthesis
- 46 Pictet-Spengler Isoquinoline Synthesis
- 47 Plancher Rearrangement
- 48 Pomeranz-Fritsch Reaction
- 49 Reissert Indole Synthesis
- 50 Reissert Reaction
- 51 Sandmeyer Isatin Synthesis
- 52 Sharpless-Katsuki Epoxidation
- 53 Skraup Reaction
- Appendix I Some Popular Name Reactions
- Appendix II Select Bibliography
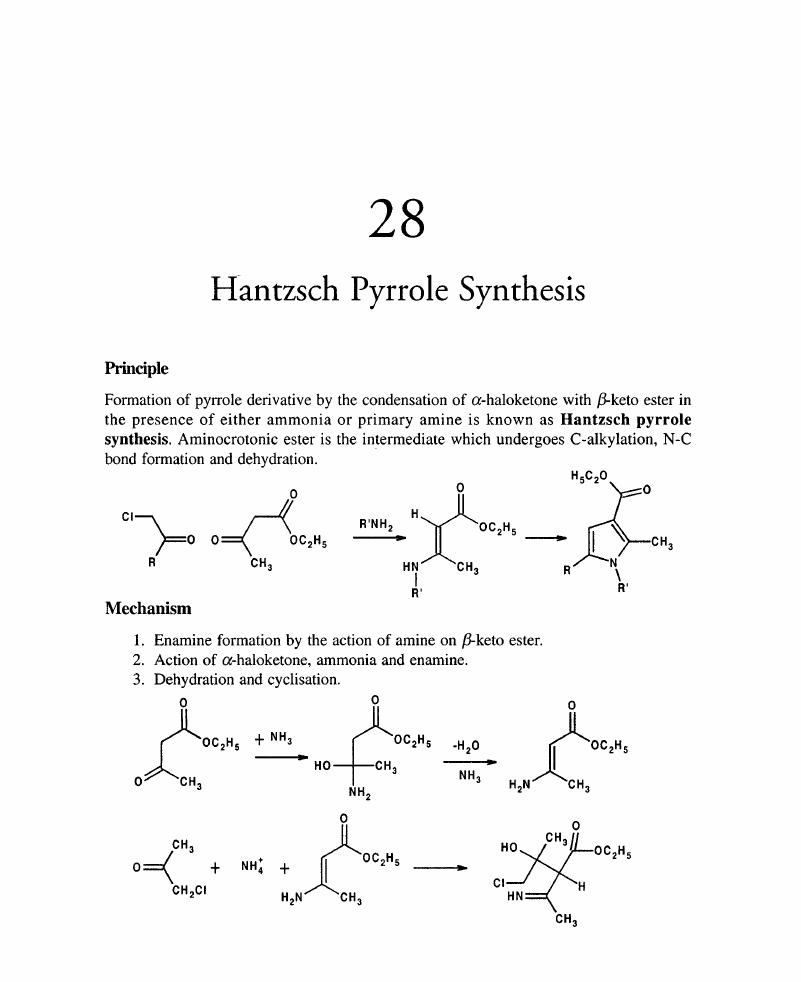
28 - Hantzsch Pyrrole Synthesis
from PART II - Heterocyclic Reactions
Published online by Cambridge University Press: 05 February 2012
- Frontmatter
- Contents
- Foreword
- Preface
- Abbreviations and Acronyms
- PART I
- PART II Heterocyclic Reactions
- 1 Algar-Flynn-Oyamada Reaction
- 2 Allan-Robinson
- 3 Auwers Synthesis
- 4 Baeyer-Drewson Indoxyl Synthesis
- 5 Bernthsen Acridine Synthesis
- 6 Betti Reaction
- 7 Biginelli Reaction
- 8 Bischler-Mohlau Indole Synthesis
- 9 Bischler-Napieralski Reaction
- 10 Borsche-Drechsel Reaction
- 11 Bucherer-Bergs Reaction
- 12 Bucherer Carbazole Synthesis
- 13 Camps Quinoline Synthesis
- 14 Chichibabin Reaction
- 15 Claisen Isoxazole Synthesis
- 16 Combes Quinoline Synthesis
- 17 Conrad-Limpach Reaction
- 18 Darzens Glycidic Ester Condensation
- 19 Doebner Reaction
- 20 Doebner-von Miller Synthesis
- 21 Erlenmeyer-Plochl Azlactone Synthesis
- 22 Fischer-Indole Synthesis
- 23 Friedlander Synthesis
- 24 Gabriel-Colman Rearrangement
- 25 Gould-Jacobs Reaction
- 26 Graebe-Ullmann Synthesis
- 27 Hantzsch Pyridine Synthesis
- 28 Hantzsch Pyrrole Synthesis
- 29 Hantzsch Thiazole, Pyridine and 1,2,4-Triazine Synthesis
- 30 Hansch Reaction (Synthesis of Benzofurans)
- 31 Hofmann-Löffler-Freytag Reaction
- 32 Knorr Pyrazole Synthesis
- 33 Knorr Pyrrole Synthesis
- 34 Knorr Quinoline Synthesis
- 35 Kostanecki-Robinson Reaction
- 36 Madelung Synthesis
- 37 Nenitzescu Indole Synthesis
- 38 Nimentowski Quinazoline Synthesis
- 39 Nimentowski Quinoline Synthesis
- 40 Paal-Knorr Synthesis
- 41 Paterno-Buchi Reaction
- 42 Payne Rearrangement
- 43 Pechmann Condensation
- 44 Pfitzinger Quinoline Synthesis
- 45 Pictet-Gams Isoquinoline Synthesis
- 46 Pictet-Spengler Isoquinoline Synthesis
- 47 Plancher Rearrangement
- 48 Pomeranz-Fritsch Reaction
- 49 Reissert Indole Synthesis
- 50 Reissert Reaction
- 51 Sandmeyer Isatin Synthesis
- 52 Sharpless-Katsuki Epoxidation
- 53 Skraup Reaction
- Appendix I Some Popular Name Reactions
- Appendix II Select Bibliography
Summary
- Type
- Chapter
- Information
- Name Reactions in Organic Synthesis , pp. 542 - 543Publisher: Foundation BooksPrint publication year: 2006

